Cancer Res Treat.
2024 Jul;56(3):785-794. 10.4143/crt.2023.1014.
Contribution of Enhanced Locoregional Control to Improved Overall Survival with Consolidative Durvalumab after Concurrent Chemoradiotherapy in Locally Advanced Non–Small Cell Lung Cancer: Insights from Real-World Data
- Affiliations
-
- 1Department of Radiation Oncology, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea
- 2Department of Radiation Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 4Department of Pulmonology and Critical Care Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2557665
- DOI: http://doi.org/10.4143/crt.2023.1014
Abstract
- Purpose
This study aimed to assess the real-world clinical outcomes of consolidative durvalumab in patients with unresectable locally advanced non–small cell lung cancer (LA-NSCLC) and to explore the role of radiotherapy in the era of immunotherapy.
Materials and Methods
This retrospective study assessed 171 patients with unresectable LA-NSCLC who underwent concurrent chemoradiotherapy (CCRT) with or without consolidative durvalumab at Asan Medical Center between May 2018 and May 2021. Primary outcomes included freedom from locoregional failure (FFLRF), distant metastasis-free survival (DMFS), progression-free survival (PFS), and overall survival (OS).
Results
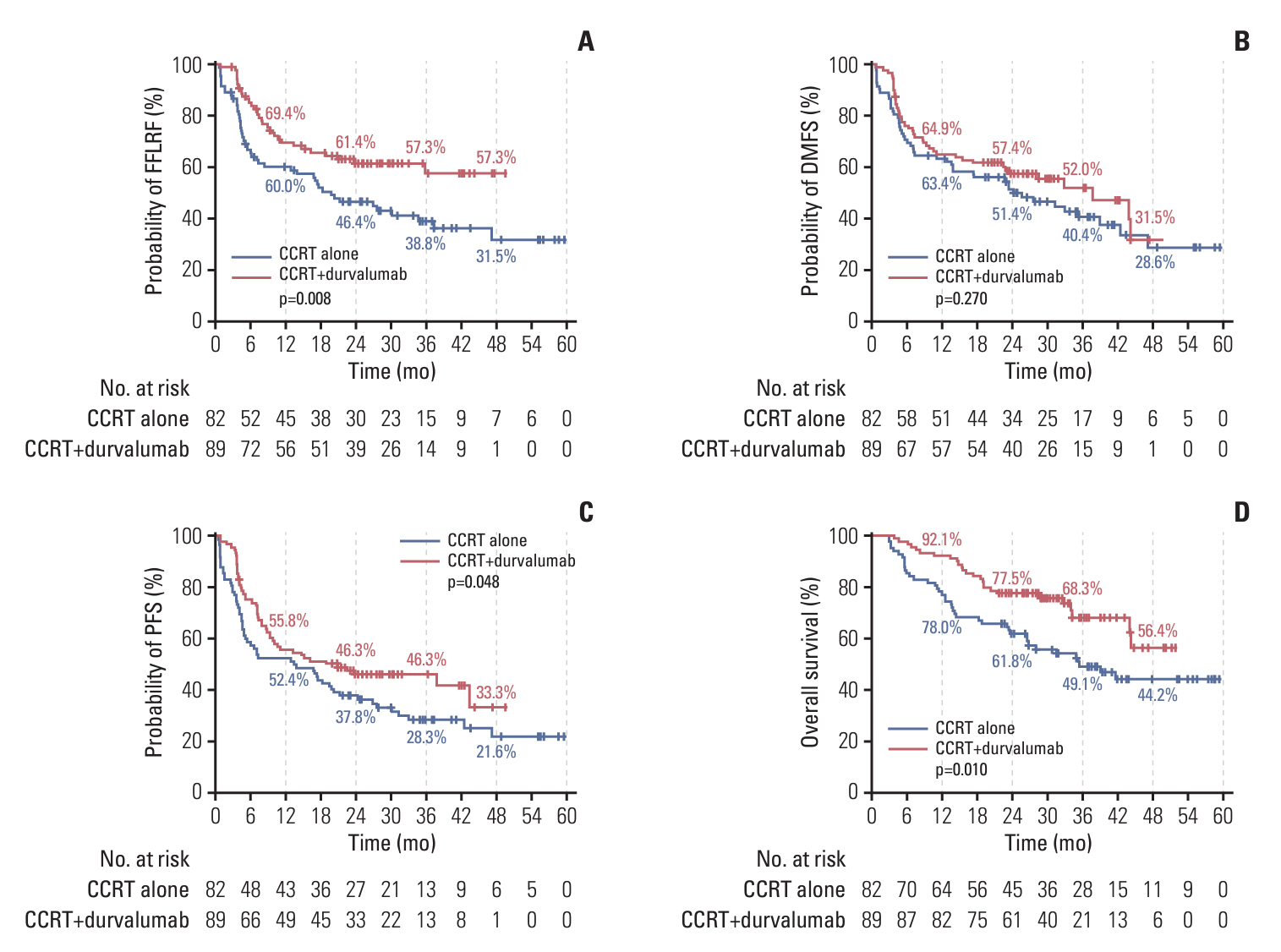
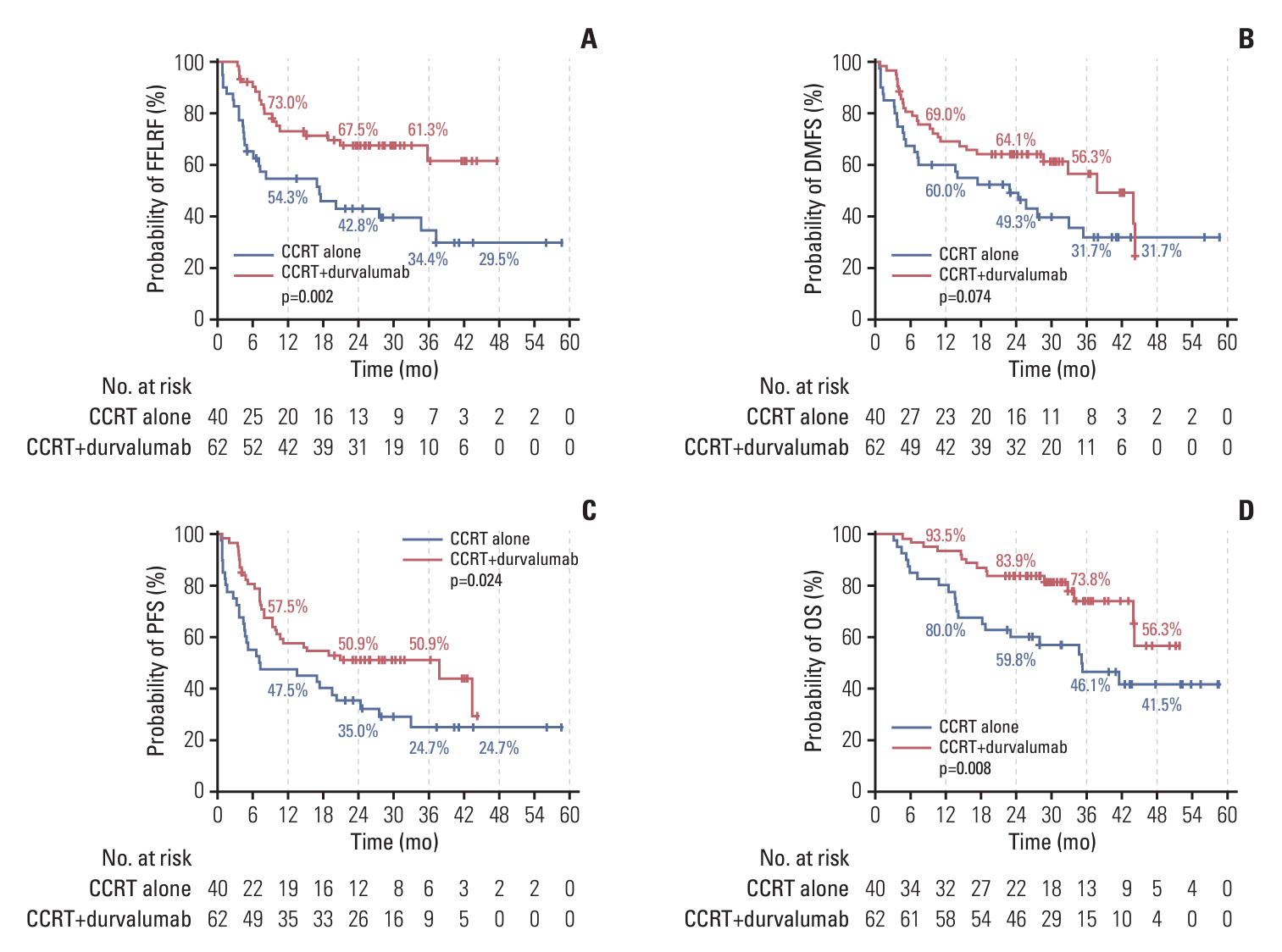
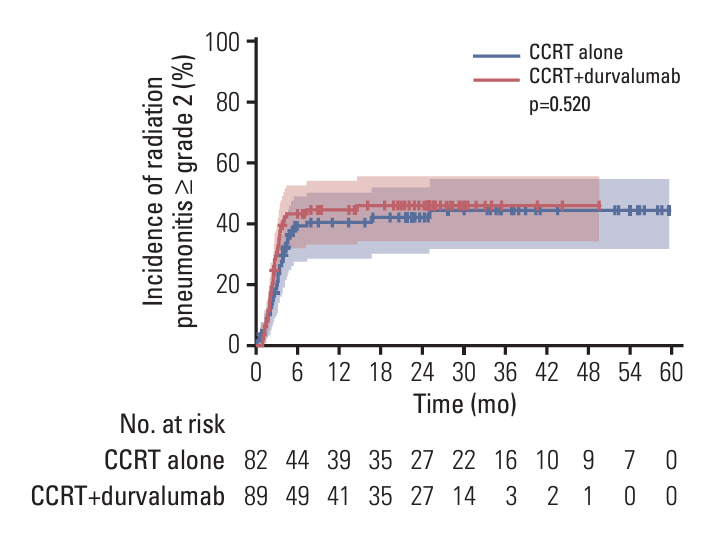
Durvalumab following CCRT demonstrated a prolonged median PFS of 20.9 months (p=0.048) and a 3-year FFLRF rate of 57.3% (p=0.008), compared to 13.7 months and 38.8%, respectively, with CCRT alone. Furthermore, the incidence of in-field recurrence was significantly greater in the CCRT-alone group compared to the durvalumab group (26.8% vs. 12.4%, p=0.027). While median OS was not reached with durvalumab, it was 35.4 months in patients receiving CCRT alone (p=0.010). Patients positive for programmed cell death ligand 1 (PD-L1) expression showed notably better outcomes, including FFLRF, DMFS, PFS, and OS. Adherence to PACIFIC trial eligibility criteria identified 100 patients (58.5%) as ineligible. The use of durvalumab demonstrated better survival regardless of eligibility criteria.
Conclusion
The use of durvalumab consolidation following CCRT significantly enhanced locoregional control and OS in patients with unresectable LA-NSCLC, especially in those with PD-L1–positive tumors, thereby validating the role of durvalumab in standard care.
Keyword
Figure
Reference
-
References
1. Thandra KC, Barsouk A, Saginala K, Aluru JS, Barsouk A. Epidemiology of lung cancer. Contemp Oncol (Pozn). 2021; 25:45–52.
Article2. Jung KW, Kang MJ, Park EH, Yun EH, Kim HJ, Kong HJ, et al. Prediction of cancer incidence and mortality in Korea, 2023. Cancer Res Treat. 2023; 55:400–7.3. Casal-Mourino A, Ruano-Ravina A, Lorenzo-Gonzalez M, Rodriguez-Martinez A, Giraldo-Osorio A, Varela-Lema L, et al. Epidemiology of stage III lung cancer: frequency, diagnostic characteristics, and survival. Transl Lung Cancer Res. 2021; 10:506–18.
Article4. Kim HC, Ji W, Lee JC, Kim HR, Song SY, Choi CM, et al. Prognostic factor and clinical outcome in stage III non-small cell lung cancer: a study based on real-world clinical data in the Korean population. Cancer Res Treat. 2021; 53:1033–41.
Article5. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017; 377:1919–29.6. Spigel DR, Faivre-Finn C, Gray JE, Vicente D, Planchard D, Paz-Ares L, et al. Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol. 2022; 40:1301–11.
Article7. Sheldrick RC. Randomized trials vs real-world evidence: how can both inform decision-making? JAMA. 2023; 329:1352–3.8. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. NCCN Guidelines(R) insights: non-small cell lung cancer, version 2.2023. J Natl Compr Canc Netw. 2023; 21:340–50.9. Jang JY, Kim SS, Song SY, Kim YJ, Kim SW, Choi EK. Radiation pneumonitis in patients with non-small-cell lung cancer receiving chemoradiotherapy and an immune checkpoint inhibitor: a retrospective study. Radiat Oncol. 2021; 16:231.
Article10. Kalbasi A, June CH, Haas N, Vapiwala N. Radiation and immunotherapy: a synergistic combination. J Clin Invest. 2013; 123:2756–63.
Article11. Mansfield AS, Park SS, Dong H. Synergy of cancer immunotherapy and radiotherapy. Aging (Albany NY). 2015; 7:144–5.
Article12. Park SS, Dong H, Liu X, Harrington SM, Krco CJ, Grams MP, et al. PD-1 restrains radiotherapy-induced abscopal effect. Cancer Immunol Res. 2015; 3:610–9.
Article13. Demaria S, Coleman CN, Formenti SC. Radiotherapy: changing the game in immunotherapy. Trends Cancer. 2016; 2:286–94.
Article14. Mielgo-Rubio X, Rojo F, Mezquita-Perez L, Casas F, Wals A, Juan M, et al. Deep diving in the PACIFIC: Practical issues in stage III non-small cell lung cancer to avoid shipwreck. World J Clin Oncol. 2020; 11:898–917.
Article15. Tachihara M, Tsujino K, Ishihara T, Hayashi H, Sato Y, Kurata T, et al. Rationale and design for a multicenter, phase II study of durvalumab plus concurrent radiation therapy in locally advanced non-small cell lung cancer: The DOLPHIN Study (WJOG11619L). Cancer Manag Res. 2021; 13:9167–73.
Article16. Zhao B, Li H, Wu J, Ma W. Durvalumab after sequential chemoradiotherapy is safe for stage III, unresectable NSCLC: results from phase 2 PACIFIC-6 trial. J Thorac Oncol. 2023; 18:e1–2.
Article17. Taugner J, Kasmann L, Eze C, Tufman A, Reinmuth N, Duell T, et al. Durvalumab after chemoradiotherapy for PD-L1 expressing inoperable stage III NSCLC leads to significant improvement of local-regional control and overall survival in the real-world setting. Cancers (Basel). 2021; 13:1613.
Article18. Abe T, Saito S, Iino M, Aoshika T, Ryuno Y, Ohta T, et al. Effect of durvalumab on local control after concurrent chemoradiotherapy for locally advanced non-small cell lung cancer in comparison with chemoradiotherapy alone. Thorac Cancer. 2021; 12:245–50.
Article19. Faivre-Finn C, Vicente D, Kurata T, Planchard D, Paz-Ares L, Vansteenkiste JF, et al. Four-year survival with durvalumab after chemoradiotherapy in stage III NSCLC: an update from the PACIFIC trial. J Thorac Oncol. 2021; 16:860–7.20. Park CK, Oh HJ, Kim YC, Kim YH, Ahn SJ, Jeong WG, et al. Korean real-world data on patients with unresectable stage III NSCLC treated with durvalumab after chemoradiotherapy: PACIFIC-KR. J Thorac Oncol. 2023; 18:1042–54.
Article21. Jung HA, Noh JM, Sun JM, Lee SH, Ahn JS, Ahn MJ, et al. Real world data of durvalumab consolidation after chemoradiotherapy in stage III non-small-cell lung cancer. Lung Cancer. 2020; 146:23–9.
Article22. Miura Y, Mouri A, Kaira K, Yamaguchi O, Shiono A, Hashimoto K, et al. Chemoradiotherapy followed by durvalumab in patients with unresectable advanced non-small cell lung cancer: management of adverse events. Thorac Cancer. 2020; 11:1280–7.
Article23. Desilets A, Blanc-Durand F, Lau S, Hakozaki T, Kitadai R, Malo J, et al. Durvalumab therapy following chemoradiation compared with a historical cohort treated with chemoradiation alone in patients with stage III non-small cell lung cancer: a real-world multicentre study. Eur J Cancer. 2021; 142:83–91.
Article24. Taugner J, Kasmann L, Eze C, Ruhle A, Tufman A, Reinmuth N, et al. Real-world prospective analysis of treatment patterns in durvalumab maintenance after chemoradiotherapy in unresectable, locally advanced NSCLC patients. Invest New Drugs. 2021; 39:1189–96.
Article25. Tsukita Y, Yamamoto T, Mayahara H, Hata A, Takeda Y, Nakayama H, et al. Intensity-modulated radiation therapy with concurrent chemotherapy followed by durvalumab for stage III non-small cell lung cancer: a multi-center retrospective study. Radiother Oncol. 2021; 160:266–72.
Article26. Guberina M, Guberina N, Pottgen C, Gauler T, Richlitzki C, Metzenmacher M, et al. Effectiveness of durvalumab consolidation in stage III non-small-cell lung cancer: focus on treatment selection and prognostic factors. Immunotherapy. 2022; 14:927–44.
Article27. Yamamoto T, Tsukita Y, Katagiri Y, Matsushita H, Umezawa R, Ishikawa Y, et al. Durvalumab after chemoradiotherapy for locally advanced non-small cell lung cancer prolonged distant metastasis-free survival, progression-free survival and overall survival in clinical practice. BMC Cancer. 2022; 22:364.
Article28. Girard N, Bar J, Garrido P, Garassino MC, McDonald F, Mornex F, et al. Treatment characteristics and real-world progression-free survival in patients with unresectable stage III NSCLC who received durvalumab after chemoradiotherapy: findings from the PACIFIC-R study. J Thorac Oncol. 2023; 18:181–93.
Article29. Mayahara H, Uehara K, Harada A, Kitatani K, Yabuuchi T, Miyazaki S, et al. Predicting factors of symptomatic radiation pneumonitis induced by durvalumab following concurrent chemoradiotherapy in locally advanced non-small cell lung cancer. Radiat Oncol. 2022; 17:7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Multidisciplinary Management of the Locally Advanced Unresectable Non-Small Cell Lung Cancer
- Concurrent Chemoradiotherapy with Weekly Paclitaxel for Locally Advanced Non-small Cell Lung Cancer
- Definitive Radiotherapy of Non-Small Cell Lung Cancer
- Current Update on the Management of Locally Advanced Non-small Cell Lung Cancer
- Management of Locally Advanced Non-small Cell Lung Cancer