Ann Surg Treat Res.
2024 Jul;107(1):50-57. 10.4174/astr.2024.107.1.50.
Scaffold-based synergistic enhancement of stem cell effects for therapeutic angiogenesis in critical limb ischemia: an experimental animal study
- Affiliations
-
- 1Department of Surgery, Seoul National University College of Medicine, Seoul, Korea
- 2Department of Surgery, Seoul National University Bundang Hospital, Seongnam, Korea
- 3Department of Pharmacology, Chung-Ang University College of Medicine, Seoul, Korea
- KMID: 2557580
- DOI: http://doi.org/10.4174/astr.2024.107.1.50
Abstract
- Purpose
Stem cell-based therapies are considered an alternative approach for critical limb ischemia (CLI) patients with limited or exhausted options, yet their clinical use is limited by the lack of sustainability and unclear mechanism of action. In this study, a substance P-conjugated scaffold was injected with mesenchymal stem cells (MSCs) into an animal model of CLI to verify whether angiogenesis could be enhanced.
Methods
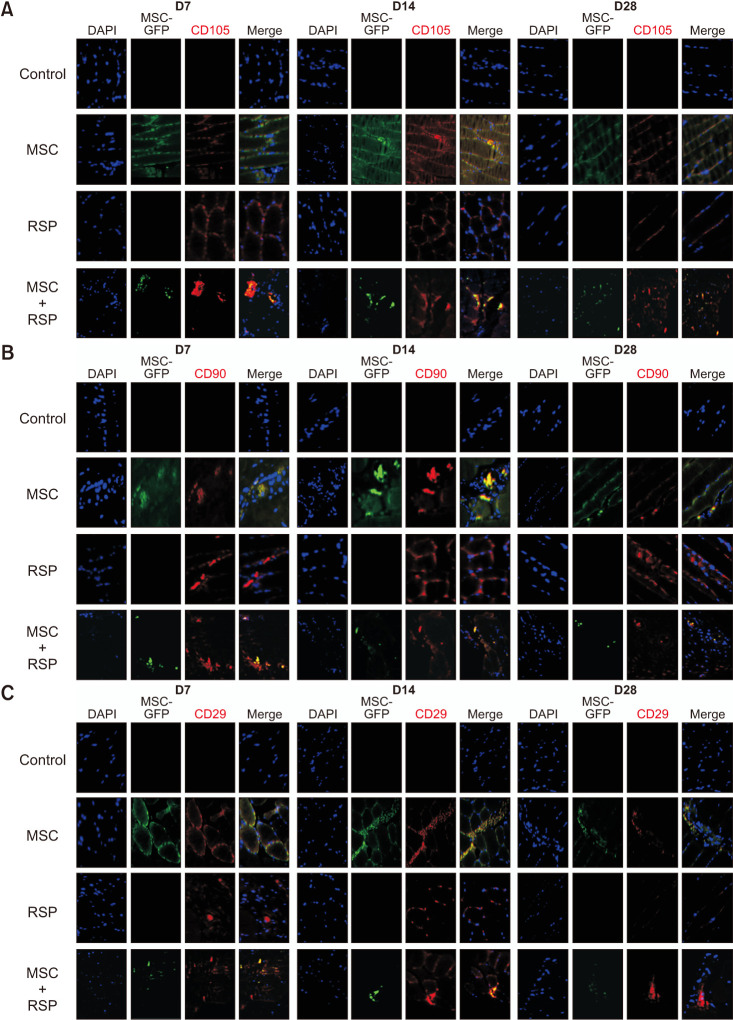
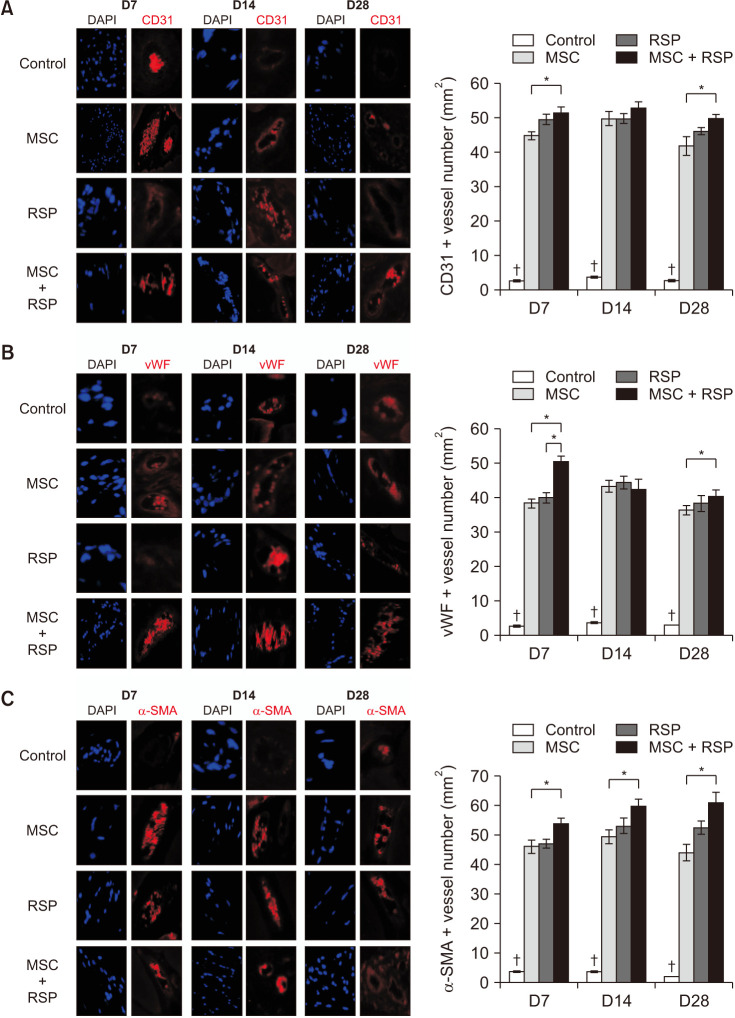
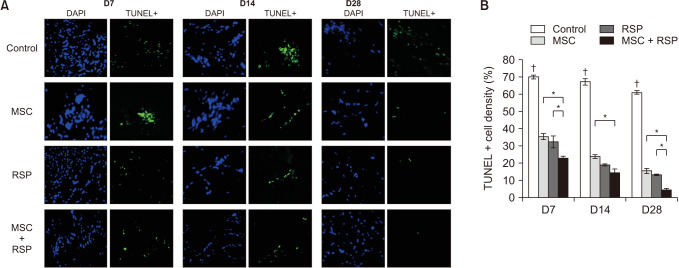
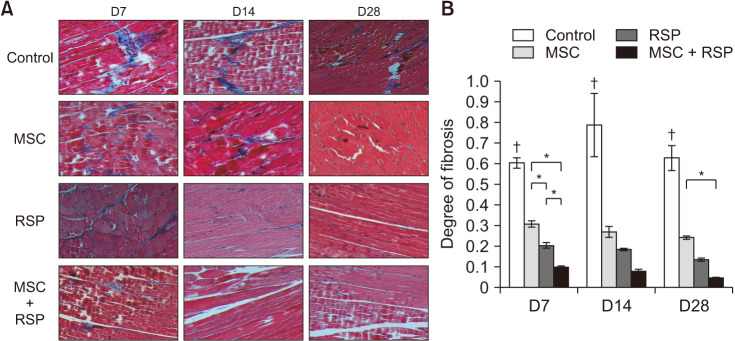
A self-assembling peptide (SAP) was conjugated with substance P, known to have the ability to recruit host stem cells into the site of action. This SAP was injected with MSCs into ischemic hindlimbs of rats, and the presence of MSCs was verified by immunohistochemical (IHC) staining of MSC-specific markers at days 7, 14, and 28. The degree of angiogenesis, cell apoptosis, and fibrosis was also quantified.
Results
Substance P-conjugated SAP was able to recruit intrinsic MSCs into the ischemic site of action. When injected in combination with MSCs, the presence of both injected and recruited MSCs was found in the ischemic tissues by double IHC staining. This in turn led to a higher degree of angiogenesis, less cell apoptosis, and less tissue fibrosis compared to the other groups at all time points.
Conclusion
The combination of substance P-conjugated SAP and MSCs was able to enhance angiogenesis and tissue repair, which was achieved by the additive effect from exogenously administered and intrinsically recruited MSCs. This scaffold-based intrinsic recruitment approach could be a viable option to enhance the therapeutic effects in patients with CLI.
Keyword
Figure
Reference
-
1. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. 2022; 145:e153–e639. PMID: 35078371.2. Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA. The global burden of cardiovascular diseases and risk: a compass for future health. J Am Coll Cardiol. 2022; 80:2361–2371. PMID: 36368511.3. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007; 45 Suppl S:S5–S67. PMID: 17223489.4. Sprengers RW, Teraa M, Moll FL, de Wit GA, van der Graaf Y, Verhaar MC, et al. Quality of life in patients with no-option critical limb ischemia underlines the need for new effective treatment. J Vasc Surg. 2010; 52:843–849. PMID: 20598482.5. Kim HJ, Jang SY, Kim JM, Kim SY, Kim BM, Kim WB, et al. Vascular endothelial growth factor (VEGF)-induced angiogenic gene therapy in a patient with critical limb ischemia. Korean J Med. 2003; 64:85–90.6. Keighron C, Lyons CJ, Creane M, O’Brien T, Liew A. Recent advances in endothelial progenitor cells toward their use in clinical translation. Front Med (Lausanne). 2018; 5:354. PMID: 30619864.7. Liew A, Bhattacharya V, Shaw J, Stansby G. Cell therapy for critical limb ischemia: a meta-analysis of randomized controlled trials. Angiology. 2016; 67:444–455. PMID: 26195561.8. Park HS, Hahn S, Choi GH, Yoo YS, Lee JY, Lee T. Muscle-derived stem cells promote angiogenesis and attenuate intimal hyperplasia in different murine vascular disease models. Stem Cells Dev. 2013; 22:866–877. PMID: 23082782.9. Park HS, Choi GH, Hahn S, Yoo YS, Lee JY, Lee T. Potential role of vascular smooth muscle cell-like progenitor cell therapy in the suppression of experimental abdominal aortic aneurysms. Biochem Biophys Res Commun. 2013; 431:326–331. PMID: 23291168.10. Zhao X, Li Q, Guo Z, Li Z. Constructing a cell microenvironment with biomaterial scaffolds for stem cell therapy. Stem Cell Res Ther. 2021; 12:583. PMID: 34809719.11. Park HS, Choi GH, Kim D, Jung TW, Jung IM, Chung JK, et al. Use of self-assembling peptides to enhance stem cell function for therapeutic angiogenesis. Stem Cells Int. 2018; 2018:4162075. PMID: 30008751.12. Zhang S, Gelain F, Zhao X. Designer self-assembling peptide nanofiber scaffolds for 3D tissue cell cultures. Semin Cancer Biol. 2005; 15:413–420. PMID: 16061392.13. Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nat Biotechnol. 2003; 21:1171–1178. PMID: 14520402.14. Kim JH, Jung Y, Kim BS, Kim SH. Stem cell recruitment and angiogenesis of neuropeptide substance P coupled with self-assembling peptide nanofiber in a mouse hind limb ischemia model. Biomaterials. 2013; 34:1657–1668. PMID: 23206876.15. Hong HS, Kim S, Jin Y, Son Y. Substance P enhances the therapeutic effect of MSCs by modulating their angiogenic potential. J Cell Mol Med. 2020; 24:12560–12571. PMID: 32985796.16. Shafiq M, Zhang Q, Zhi D, Wang K, Kong D, Kim DH, et al. In situ blood vessel regeneration using SP (Substance P) and SDF (Stromal Cell-Derived Factor)-1α peptide eluting vascular grafts. Arterioscler Thromb Vasc Biol. 2018; 38:e117–e134. PMID: 29853570.17. Zhang Y, An S, Hao J, Tian F, Fang X, Wang J. Systemic injection of substance p promotes murine calvarial repair through mobilizing endogenous mesenchymal stem cells. Sci Rep. 2018; 8:12996. PMID: 30158583.18. Kim JH, Jung Y, Kim SH, Sun K, Choi J, Kim HC, et al. The enhancement of mature vessel formation and cardiac function in infarcted hearts using dual growth factor delivery with self-assembling peptides. Biomaterials. 2011; 32:6080–6088. PMID: 21636123.19. Perin E, Loveland L, Caporusso J, Dove C, Motley T, Sigal F, et al. Gene therapy for diabetic foot ulcers: interim analysis of a randomised, placebo-controlled phase 3 study of VM202 (ENGENSIS), a plasmid DNA expressing two isoforms of human hepatocyte growth factor. Int Wound J. 2023; 20:3531–3539. PMID: 37230802.20. Wahid FS, Ismail NA, Wan Jamaludin WF, Muhamad NA, Mohamad Idris MA, Lai NM. Efficacy and safety of autologous cell-based therapy in patients with no-option critical limb ischaemia: a meta-analysis. Curr Stem Cell Res Ther. 2018; 13:265–283. PMID: 29532760.21. Yanlian Y, Ulung K, Xiumei W, Horii A, Yokoi H, Shuguang Z. Designer self-assembling peptide nanomaterials. Nano Today. 2009; 4:193–210.22. Liu X, Wang X, Wang X, Ren H, He J, Qiao L, et al. Functionalized self-assembling peptide nanofiber hydrogels mimic stem cell niche to control human adipose stem cell behavior in vitro. Acta Biomater. 2013; 9:6798–6805. PMID: 23380207.23. Stevenson MD, Piristine H, Hogrebe NJ, Nocera TM, Boehm MW, Reen RK, et al. A self-assembling peptide matrix used to control stiffness and binding site density supports the formation of microvascular networks in three dimensions. Acta Biomater. 2013; 9:7651–7661. PMID: 23603000.24. Mistrova E, Kruzliak P, Chottova Dvorakova M. Role of substance P in the cardiovascular system. Neuropeptides. 2016; 58:41–51. PMID: 26706184.25. Steinhoff MS, von Mentzer B, Geppetti P, Pothoulakis C, Bunnett NW. Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiol Rev. 2014; 94:265–301. PMID: 24382888.26. Garcia-Recio S, Gascón P. Biological and pharmacological aspects of the NK1-receptor. Biomed Res Int. 2015; 2015:495704. PMID: 26421291.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Therapeutic Potential of Human Mesenchymal Stem Cells for Treating Ischemic Limb Diseases
- Therapeutic Angiogenesis with Somatic Stem Cell Transplantation
- The Study of BD-MSC Therapy against Critical Limb Ischemia
- Increasing injection frequency enhances the survival of injected bone marrow derived mesenchymal stem cells in a critical limb ischemia animal model
- Therapeutic Angiogenesis: The Pros and Cons and the Future