Kosin Med J.
2024 Mar;39(1):51-59. 10.7180/kmj.24.101.
Clinical efficacy and safety of autologous serum intramuscular injection in patients with mild-to-moderate atopic dermatitis: a prospective, open-label, uncontrolled study
- Affiliations
-
- 1Department of Internal Medicine, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea
- 2Department of Dermatology, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea
- 3Department of Molecular Biology and Immunology, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea
- KMID: 2556798
- DOI: http://doi.org/10.7180/kmj.24.101
Abstract
- Background
Autologous blood therapy (ABT) has been used to treat atopic dermatitis (AD) for over a century, even though evidence supporting its efficacy is lacking. We aimed to investigate the effectiveness and safety of autologous serum intramuscular injection (ASIM), which is a modified form of ABT, in treating mild-to-moderate AD.
Methods
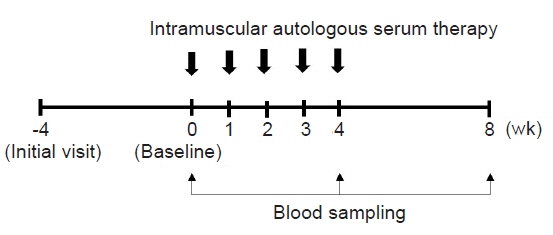
This study was a 12-week, open-label, prospective, uncontrolled trial. Following a 4-week run-in period, 22 out of 25 screened patients received ASIM once a week for 4 weeks in conjunction with standard treatment. The primary outcome measure was the Eczema Area and Severity Index (EASI), while the secondary outcomes included the Scoring Atopic Dermatitis (SCORAD) score, Dermatologic Life Quality Index (DLQI), and patient ratings of pruritus, sleep difficulty, disease status, and treatment effectiveness. Safety parameters were also assessed.
Results
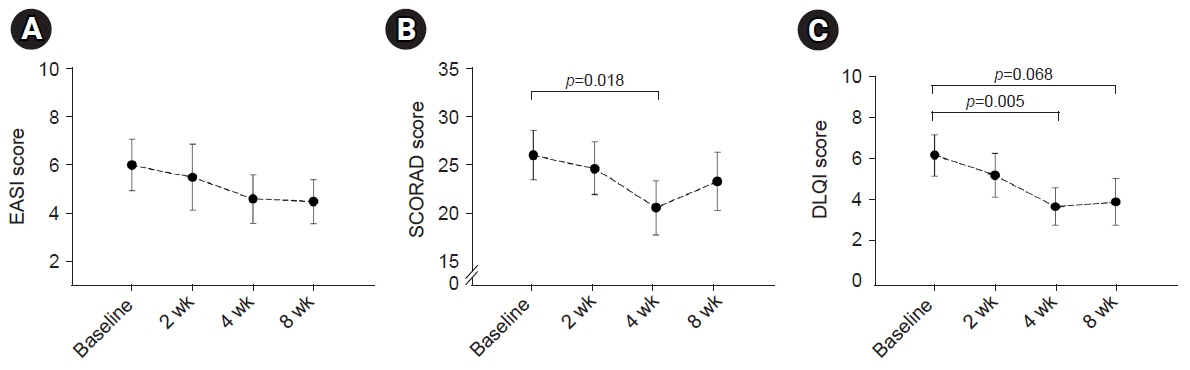
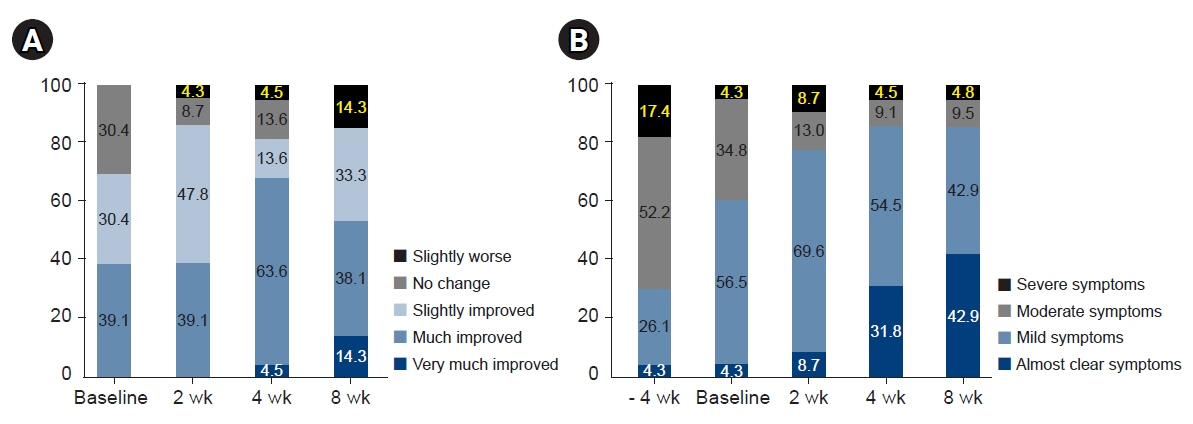
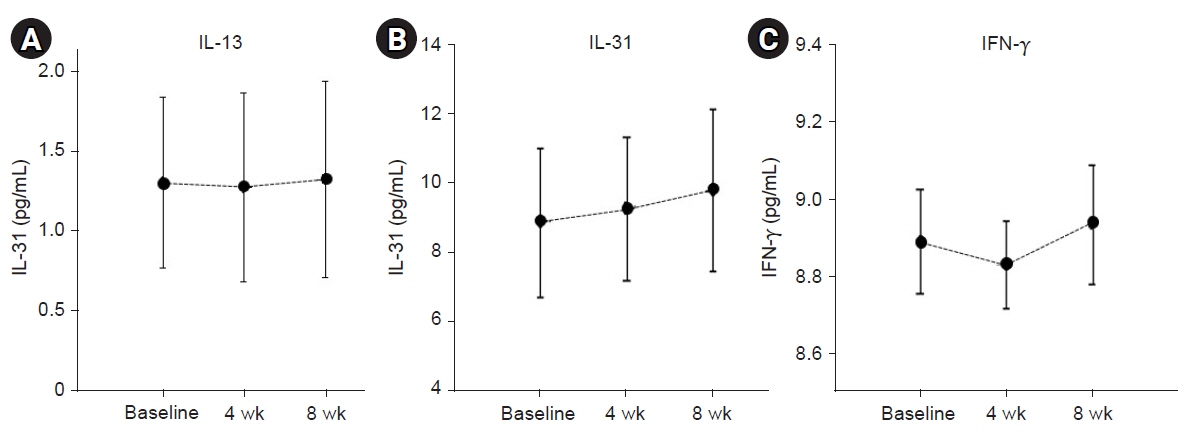
EASI scores showed a non-statistically significant trend toward improvement during ASIM intervention. Patients with at least a 50% improvement in the EASI score at 4 weeks were older and had lower peripheral eosinophil counts (p<0.05). Secondary endpoints, including the SCORAD score, pruritus, sleep difficulty, and DLQI, demonstrated statistically significant improvements at week 4 compared to baseline (p<0.05). No significant adverse reactions were observed.
Conclusions
This pioneering study suggests that repeated ASIM may improve the clinical symptoms of mild-to-moderate AD, particularly in terms of pruritus and overall quality of life. However, further research with a larger sample size is required to establish the clinical significance of these findings.
Figure
Reference
-
References
1. Stander S. Atopic dermatitis. N Engl J Med. 2021; 384:1136–43.2. Darsow U, Wollenberg A, Simon D, Taieb A, Werfel T, Oranje A, et al. ETFAD/EADV eczema task force 2009 position paper on diagnosis and treatment of atopic dermatitis. J Eur Acad Dermatol Venereol. 2010; 24:317–28.3. Schafer T. Epidemiology of complementary alternative medicine for asthma and allergy in Europe and Germany. Ann Allergy Asthma Immunol. 2004; 93(2 Suppl 1):S5–10.4. Oomen-Welke K, Huber R. Intramuscular autologous blood therapy: a systematic review of controlled trials. BMC Complement Altern Med. 2019; 19:248.5. Schafer T, Riehle A, Wichmann HE, Ring J. Alternative medicine in allergies - prevalence, patterns of use, and costs. Allergy. 2002; 57:694–700.6. Pittler MH, Armstrong NC, Cox A, Collier PM, Hart A, Ernst E. Randomized, double-blind, placebo-controlled trial of autologous blood therapy for atopic dermatitis. Br J Dermatol. 2003; 148:307–13.7. Bajaj AK, Saraswat A, Upadhyay A, Damisetty R, Dhar S. Autologous serum therapy in chronic urticaria: old wine in a new bottle. Indian J Dermatol Venereol Leprol. 2008; 74:109–13.8. Godse KV, Nadkarni N, Patil S, Mehta A. Subcutaneous autologous serum therapy in chronic spontaneous urticaria. Indian J Dermatol. 2017; 62:505–7.9. Cho SM, Kim ME, Kim JY, Park JC, Nahm DH. Clinical efficacy of autologous plasma therapy for atopic dermatitis. Dermatology. 2014; 228:71–7.10. Lee HS. Ethical issues in clinical research and publication. Kosin Med J. 2022; 37:278–82.11. Kim DJ, Kil SY, Son J, Lee HS. How to conduct well-designed clinical research. Kosin Med J. 2022; 37:187–91.12. Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol. 2001; 10:11–8.13. Oranje AP, Glazenburg EJ, Wolkerstorfer A, de Waard-van der Spek FB. Practical issues on interpretation of Scoring Atopic Dermatitis: the SCORAD index, objective SCORAD and the three-item severity score. Br J Dermatol. 2007; 157:645–8.14. Futamura M, Leshem YA, Thomas KS, Nankervis H, Williams HC, Simpson EL. A systematic review of Investigator Global Assessment (IGA) in atopic dermatitis (AD) trials: many options, no standards. J Am Acad Dermatol. 2016; 74:288–94.15. Basra MK, Salek MS, Camilleri L, Sturkey R, Finlay AY. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology. 2015; 230:27–33.16. Chopra R, Silverberg JI. Assessing the severity of atopic dermatitis in clinical trials and practice. Clin Dermatol. 2018; 36:606–15.17. Puar N, Chovatiya R, Paller AS. New treatments in atopic dermatitis. Ann Allergy Asthma Immunol. 2021; 126:21–31.18. Nahm DH. Personalized immunomodulatory therapy for atopic dermatitis: an allergist's view. Ann Dermatol. 2015; 27:355–63.19. Bielory L. Complementary and alternative interventions in asthma, allergy, and immunology. Ann Allergy Asthma Immunol. 2004; 93(2 Suppl 1):S45–54.20. Datta A, Chandra S, Saha A, Sil A, Das NK. Exploring the safety and effectiveness of subcutaneous autologous serum therapy versus conventional intramuscular autologous serum therapy in chronic urticaria: an observer-blind, randomized, controlled study. Indian J Dermatol Venereol Leprol. 2020; 86:632–42.21. Staubach P, Onnen K, Vonend A, Metz M, Siebenhaar F, Tschentscher I, et al. Autologous whole blood injections to patients with chronic urticaria and a positive autologous serum skin test: a placebo-controlled trial. Dermatology. 2006; 212:150–9.22. Debbarman P, Sil A, Datta PK, Bandyopadhyay D, Das NK. Autologous serum therapy in chronic urticaria: a promising complement to antihistamines. Indian J Dermatol. 2014; 59:375–82.23. Kocaturk E, Aktas S, Turkoglu Z, Kavala M, Zindanci I, Koc M, et al. Autologous whole blood and autologous serum injections are equally effective as placebo injections in reducing disease activity in patients with chronic spontaneous urticaria: a placebo controlled, randomized, single-blind study. J Dermatolog Treat. 2012; 23:465–71.24. Ravaut P. Essay on autohemotherapy in some dermatoses. Ann Dermatol Syphiligr. 1913; 4:292–6.25. Piconi S, Trabattoni D, Iemoli E, Fusi ML, Villa ML, Milazzo F, et al. Immune profiles of patients with chronic idiopathic urticaria. Int Arch Allergy Immunol. 2002; 128:59–66.26. Brewer DD. A systematic review of autohemotherapy as a treatment for urticaria and eczema. Cureus. 2014; 6:e233.27. Zeng ZW, Huang JQ, Chen Y, Yu X, Zhu W, Zhang DS. Acupoint autohemotherapy attenuates atopic dermatitis lesions by regulating Th1/Th2 balance in DNCB-induced BALB/c mice. Chin J Integr Med. 2022; 28:612–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intramuscular Injection of Autologous Serum in Adolescent and Adult Patients with Atopic Dermatitis: A Preliminary Randomized Clinical Trial
- What are the clinical usefulness and scientific value of intramuscular injection of autologous serum (autologous serum therapy) in patients with atopic dermatitis?
- Tacrolimus ointment: An Open study for Effects on Severe Facial Atopic Dermatitis in Korean
- Autologous Immunoglobulin Therapy in Patients With Severe Recalcitrant Atopic Dermatitis: A Preliminary Report
- Serum IgE Level in Patients of Atopic Dermatitis and Atopic Dermatitis with Molluscum Contagiosum