J Korean Med Sci.
2024 Jun;39(24):e190. 10.3346/jkms.2024.39.e190.
Cardiovascular Safety of COVID-19 Vaccination in Patients With Cancer: A Self-Controlled Case Series Study in Korea
- Affiliations
-
- 1School of Pharmacy, Sungkyunkwan University, Suwon, Korea
- 2Department of Development, SK Bioscience, Seongnam, Korea
- 3Harvard-MIT Center for Regulatory Science, Harvard Medical School, Boston, MA, USA
- 4Department of Biohealth Regulatory Science, Sungkyunkwan University, Suwon, Korea
- 5Department of Neurology, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin, Korea
- 6Samsung Advanced Institute for Health Sciences & Technology, Sungkyunkwan University, Seoul, Korea
- KMID: 2556686
- DOI: http://doi.org/10.3346/jkms.2024.39.e190
Abstract
- Background
Cancer patients have an increased risk of cardiovascular outcomes and are susceptible to coronavirus disease 2019 (COVID-19) infection. We aimed to assess the cardiovascular safety of COVID-19 vaccination for cancer patients in South Korea.
Methods
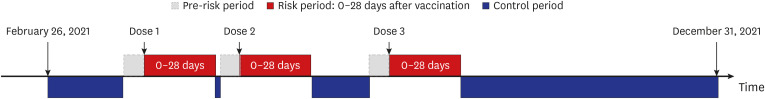
We conducted a self-controlled case series study using the K-COV-N cohort (2018– 2021). Patients with cancer aged 12 years or older who experienced cardiovascular outcomes were identified. Cardiovascular outcomes were defined as myocardial infarction, stroke, venous thromboembolism (VTE), myocarditis, or pericarditis, and the risk period was 0–28 days after receiving each dose of COVID-19 vaccines. A conditional Poisson regression model was used to calculate the incidence rate ratio (IRR) with 95% confidence interval (CI).
Results
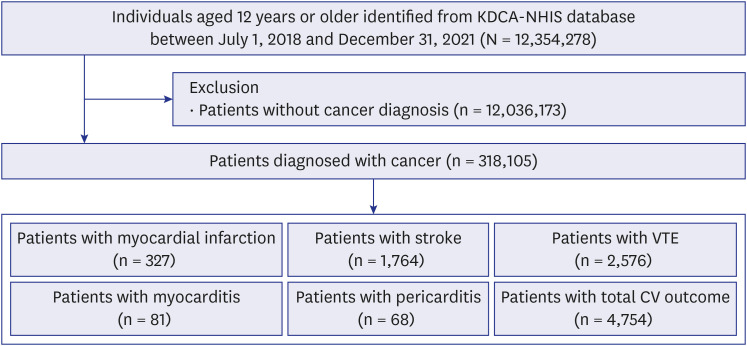
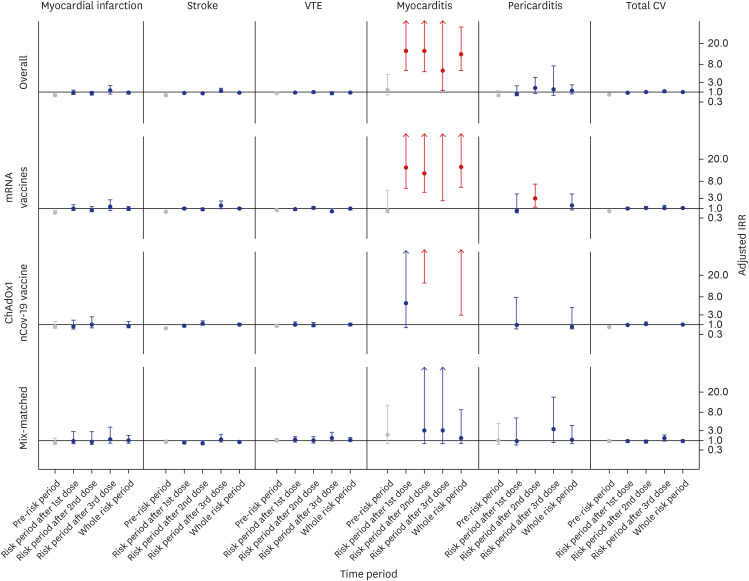
Among 318,105 patients with cancer, 4,754 patients with cardiovascular outcomes were included. The overall cardiovascular risk was not increased (adjusted IRR, 0.99 [95% CI, 0.90–1.08]) during the whole risk period. The adjusted IRRs of total cardiovascular outcomes during the whole risk period according to the vaccine type were 1.07 (95% CI, 0.95–1.21) in the mRNA vaccine subgroup, 0.99 (95% CI, 0.83–1.19) in the ChAdOx1 nCoV-19 vaccine subgroup, and 0.86 (95% CI, 0.68–1.10) in the mix-matched vaccination subgroup. However, in the analysis of individual outcome, the adjusted IRR of myocarditis was increased to 11.71 (95% CI, 5.88–23.35) during the whole risk period. In contrast, no increased risk was observed for other outcomes, such as myocardial infarction, stroke, VTE, and pericarditis.
Conclusion
For cancer patients, COVID-19 vaccination demonstrated an overall safe profile in terms of cardiovascular outcomes. However, caution is required as an increased risk of myocarditis following COVID-19 vaccination was observed in this study.
Figure
Reference
-
1. Henley SJ, Dowling NF, Ahmad FB, Ellington TD, Wu M, Richardson LC. COVID-19 and other underlying causes of cancer deaths - United States, January 2018-July 2022. MMWR Morb Mortal Wkly Rep. 2022; 71(50):1583–1588. PMID: 36520660.
Article2. Park JM, Koo HY, Lee JR, Lee H, Lee JY. COVID-19 mortality and severity in cancer patients and cancer survivors. J Korean Med Sci. 2024; 39(2):e6. PMID: 38225782.
Article3. Trapani D, Curigliano G. COVID-19 vaccines in patients with cancer. Lancet Oncol. 2021; 22(6):738–739. PMID: 34087120.
Article4. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021; 384(22):2092–2101. PMID: 33835769.
Article5. Whiteley WN, Ip S, Cooper JA, Bolton T, Keene S, Walker V, et al. Association of COVID-19 vaccines ChAdOx1 and BNT162b2 with major venous, arterial, or thrombocytopenic events: a population-based cohort study of 46 million adults in England. PLoS Med. 2022; 19(2):e1003926. PMID: 35192597.
Article6. Husby A, Hansen JV, Fosbøl E, Thiesson EM, Madsen M, Thomsen RW, et al. SARS-CoV-2 vaccination and myocarditis or myopericarditis: population based cohort study. BMJ. 2021; 375:e068665. PMID: 34916207.
Article7. Perry RJ, Tamborska A, Singh B, Craven B, Marigold R, Arthur-Farraj P, et al. Cerebral venous thrombosis after vaccination against COVID-19 in the UK: a multicentre cohort study. Lancet. 2021; 398(10306):1147–1156. PMID: 34370972.
Article8. Schultz NH, Sørvoll IH, Michelsen AE, Munthe LA, Lund-Johansen F, Ahlen MT, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021; 384(22):2124–2130. PMID: 33835768.
Article9. Wong HL, Hu M, Zhou CK, Lloyd PC, Amend KL, Beachler DC, et al. Risk of myocarditis and pericarditis after the COVID-19 mRNA vaccination in the USA: a cohort study in claims databases. Lancet. 2022; 399(10342):2191–2199. PMID: 35691322.
Article10. Jiang J, Chan L, Kauffman J, Narula J, Charney AW, Oh W, et al. Impact of vaccination on major adverse cardiovascular events in patients with COVID-19 infection. J Am Coll Cardiol. 2023; 81(9):928–930. PMID: 36813689.
Article11. Fernandes CJ, Morinaga LT, Alves JL Jr, Castro MA, Calderaro D, Jardim CV, et al. Cancer-associated thrombosis: the when, how and why. Eur Respir Rev. 2019; 28(151):28.
Article12. Fendler A, de Vries EG, GeurtsvanKessel CH, Haanen JB, Wörmann B, Turajlic S, et al. COVID-19 vaccines in patients with cancer: immunogenicity, efficacy and safety. Nat Rev Clin Oncol. 2022; 19(6):385–401. PMID: 35277694.
Article13. Ministry of Food and Drug Safety (KR). Pfizer COVID-19 vaccine age for vaccination ‘expanded’ to 12 years. Updated 2021. Accessed July 16, 2021. https://mfds.go.kr/brd/m_99/view.do?seq=45566&srchFr=&srchTo=&srchWord=&srchTp=0&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&Data_stts_gubun=C1009&page=8 .14. Nham E, Song JY, Noh JY, Cheong HJ, Kim WJ. COVID-19 vaccination in Korea: past, present, and the way forward. J Korean Med Sci. 2022; 37(47):e351. PMID: 36472087.
Article15. Waldhorn I, Holland R, Goshen-Lago T, Shirman Y, Szwarcwort-Cohen M, Reiner-Benaim A, et al. Six-month efficacy and toxicity profile of BNT162b2 vaccine in cancer patients with solid tumors. Cancer Discov. 2021; 11(10):2430–2435. PMID: 34475136.
Article16. Javadinia SA, Alizadeh K, Mojadadi MS, Nikbakht F, Dashti F, Joudi M, et al. COVID-19 vaccination in patients with malignancy; a systematic review and meta-analysis of the efficacy and safety. Front Endocrinol (Lausanne). 2022; 13:860238. PMID: 35586627.
Article17. Kuderer NM, Choueiri TK, Shah DP, Shyr Y, Rubinstein SM, Rivera DR, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020; 395(10241):1907–1918. PMID: 32473681.18. Hwang JK, Zhang T, Wang AZ, Li Z. COVID-19 vaccines for patients with cancer: benefits likely outweigh risks. J Hematol Oncol. 2021; 14(1):38. PMID: 33640005.
Article19. Pinato DJ, Tabernero J, Bower M, Scotti L, Patel M, Colomba E, et al. Prevalence and impact of COVID-19 sequelae on treatment and survival of patients with cancer who recovered from SARS-CoV-2 infection: evidence from the OnCovid retrospective, multicentre registry study. Lancet Oncol. 2021; 22(12):1669–1680. PMID: 34741822.20. Lozahic C, Maddock H, Sandhu H. Anti-cancer therapy leads to increased cardiovascular susceptibility to COVID-19. Front Cardiovasc Med. 2021; 8:634291. PMID: 33969006.
Article21. Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020; 21(3):335–337. PMID: 32066541.
Article22. Burn E, Li X, Delmestri A, Jones N, Duarte-Salles T, Reyes C, et al. Thrombosis and thrombocytopenia after vaccination against and infection with SARS-CoV-2 in the United Kingdom. Nat Commun. 2022; 13(1):7167. PMID: 36418291.
Article23. Simone A, Herald J, Chen A, Gulati N, Shen AY, Lewin B, et al. Acute myocarditis following COVID-19 mRNA vaccination in adults aged 18 years or older. JAMA Intern Med. 2021; 181(12):1668–1670. PMID: 34605853.
Article24. Le Vu S, Bertrand M, Jabagi MJ, Botton J, Drouin J, Baricault B, et al. Age and sex-specific risks of myocarditis and pericarditis following COVID-19 messenger RNA vaccines. Nat Commun. 2022; 13(1):3633. PMID: 35752614.
Article25. Bae DH, Kim M, Lee DI, Lee JH, Kim S, Lee SY, et al. Simultaneous occurrence of immune-mediated thrombocytopenia and myocarditis after mRNA-1273 COVID-19 vaccination: a case report. J Korean Med Sci. 2022; 37(21):e169. PMID: 35638196.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Aphthous Stomatitis in a Healthy Adult Following COVID-19 Vaccination: Clinical Reasoning
- Vaccination Rates of Hospitalized Patients at High Risk of Severe COVID-19: A Single-Center CrossSectional Study
- COVID-19 vaccination–related cardiovascular complications
- COVID-19 Vaccination in Korea
- The Role of COVID-19 Vaccination for Patients With Atherosclerotic Cardiovascular Disease in the Upcoming Endemic Era