Endocrinol Metab.
2024 Jun;39(3):500-510. 10.3803/EnM.2023.1860.
End-to-End Semi-Supervised Opportunistic Osteoporosis Screening Using Computed Tomography
- Affiliations
-
- 1Healthcare AI Team, National Cancer Center, Goyang, Korea
- 2Department of Bio and Brain Engineering, Korea Advanced Institute of Science and Technology (KAIST), Daejeon, Korea
- 3Kim Jaechul Graduate School of AI, Korea Advanced Institute of Science and Technology (KAIST), Daejeon, Korea
- KMID: 2556642
- DOI: http://doi.org/10.3803/EnM.2023.1860
Abstract
- Background
Osteoporosis is the most common metabolic bone disease and can cause fragility fractures. Despite this, screening utilization rates for osteoporosis remain low among populations at risk. Automated bone mineral density (BMD) estimation using computed tomography (CT) can help bridge this gap and serve as an alternative screening method to dual-energy X-ray absorptiometry (DXA).
Methods
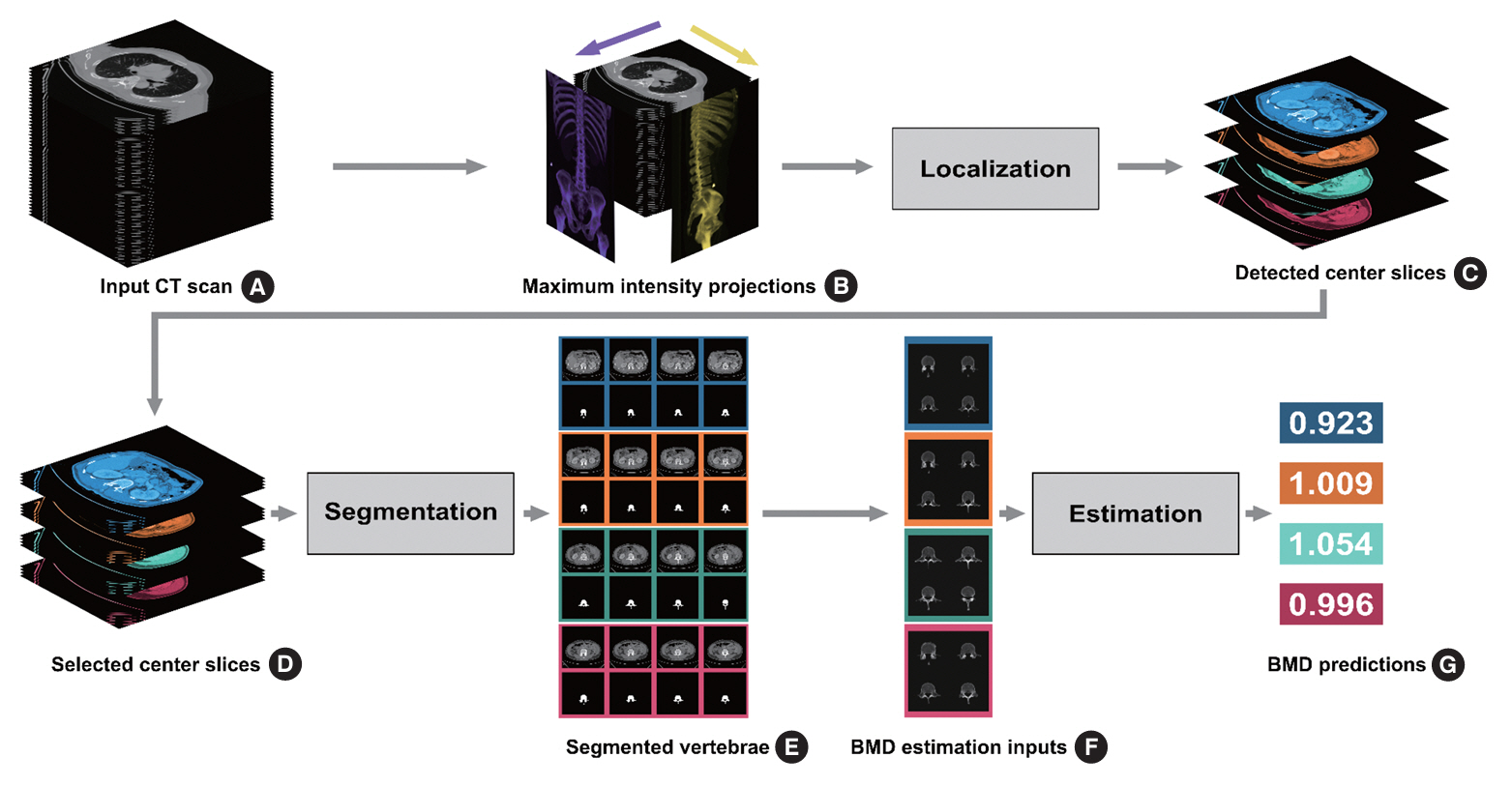
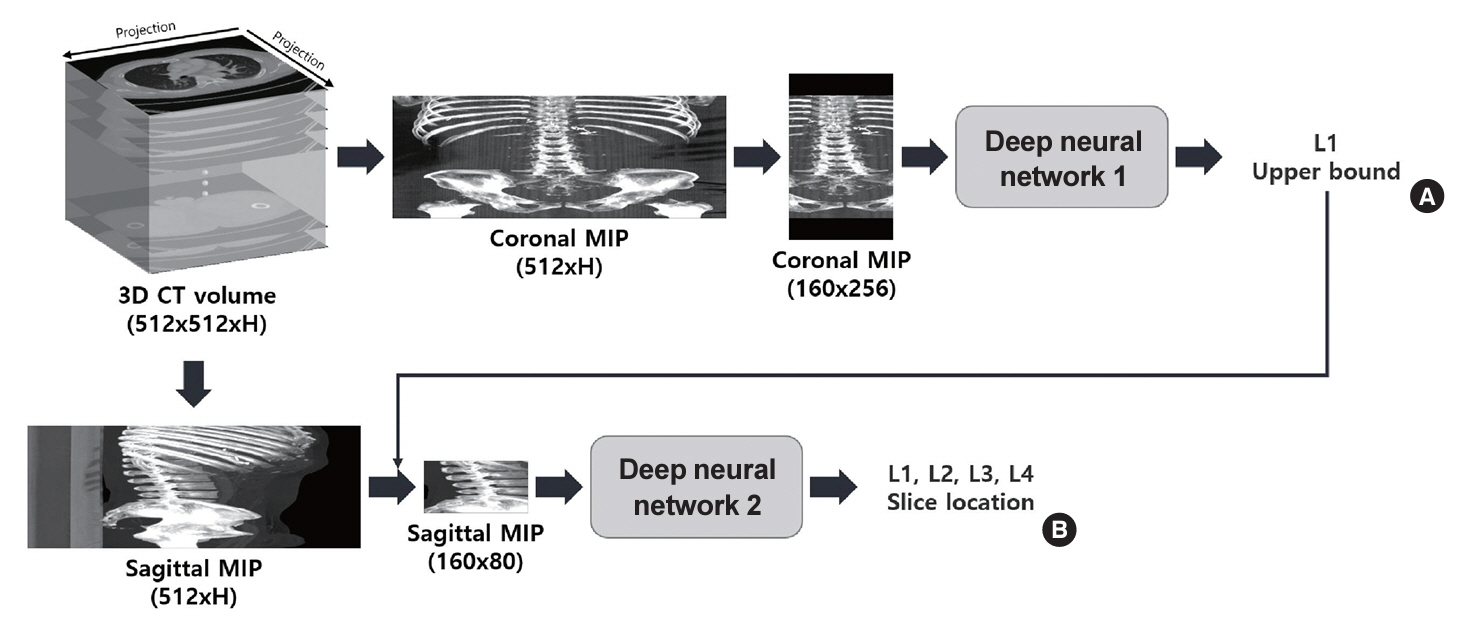
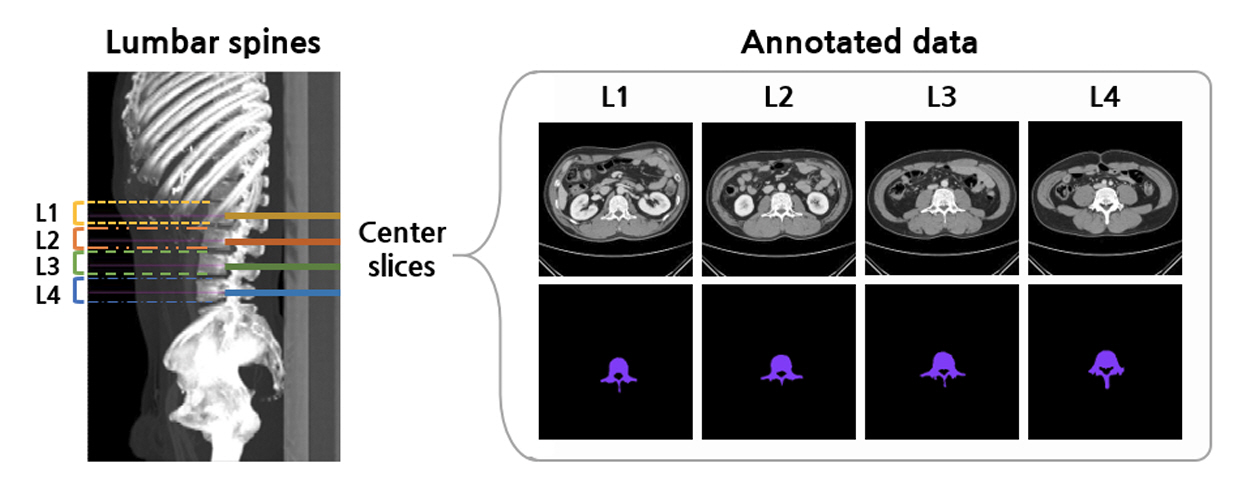
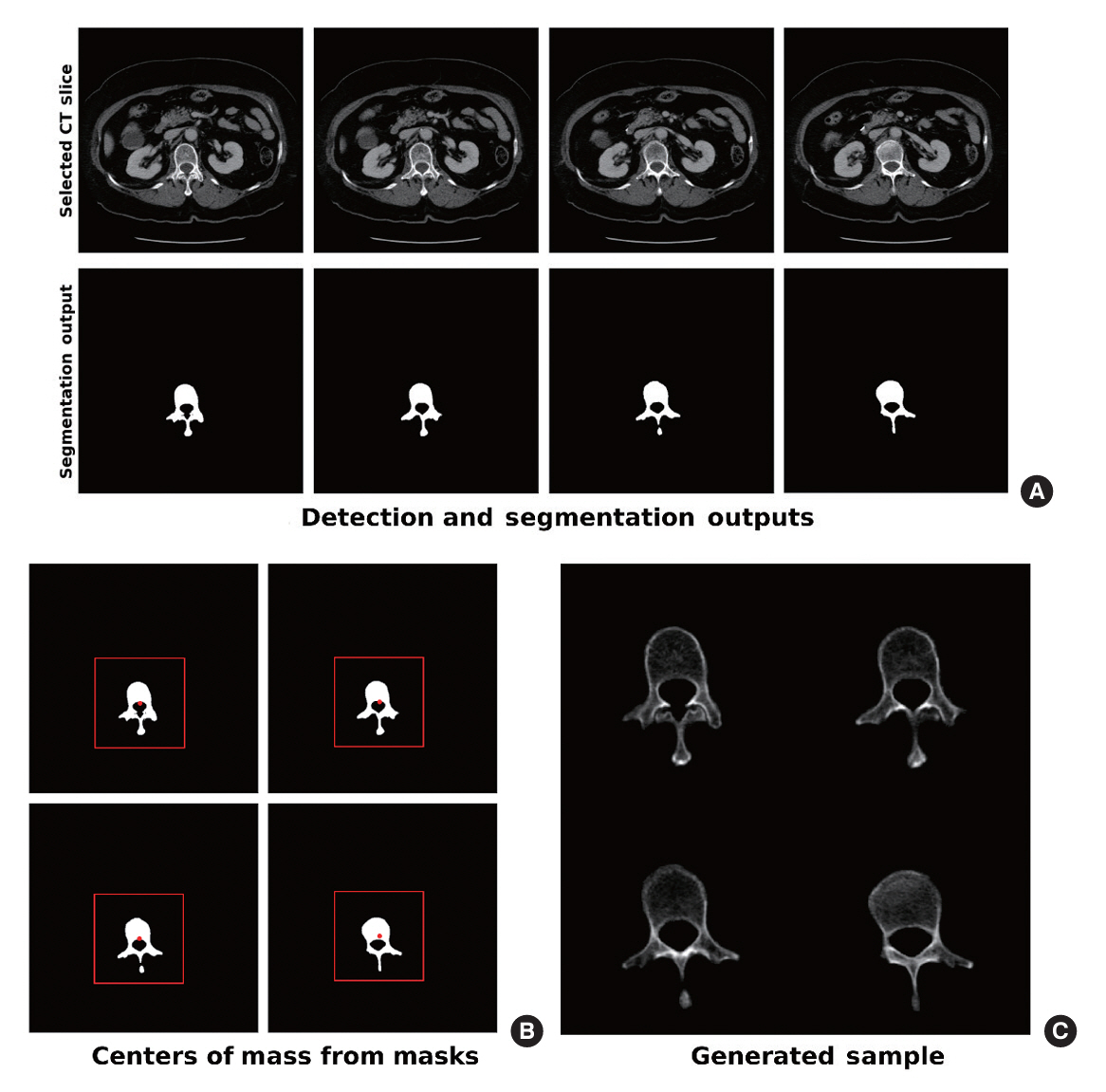
The feasibility of an opportunistic and population agnostic screening method for osteoporosis using abdominal CT scans without bone densitometry phantom-based calibration was investigated in this retrospective study. A total of 268 abdominal CT-DXA pairs and 99 abdominal CT studies without DXA scores were obtained from an oncology specialty clinic in the Republic of Korea. The center axial CT slices from the L1, L2, L3, and L4 lumbar vertebrae were annotated with the CT slice level and spine segmentation labels for each subject. Deep learning models were trained to localize the center axial slice from the CT scan of the torso, segment the vertebral bone, and estimate BMD for the top four lumbar vertebrae.
Results
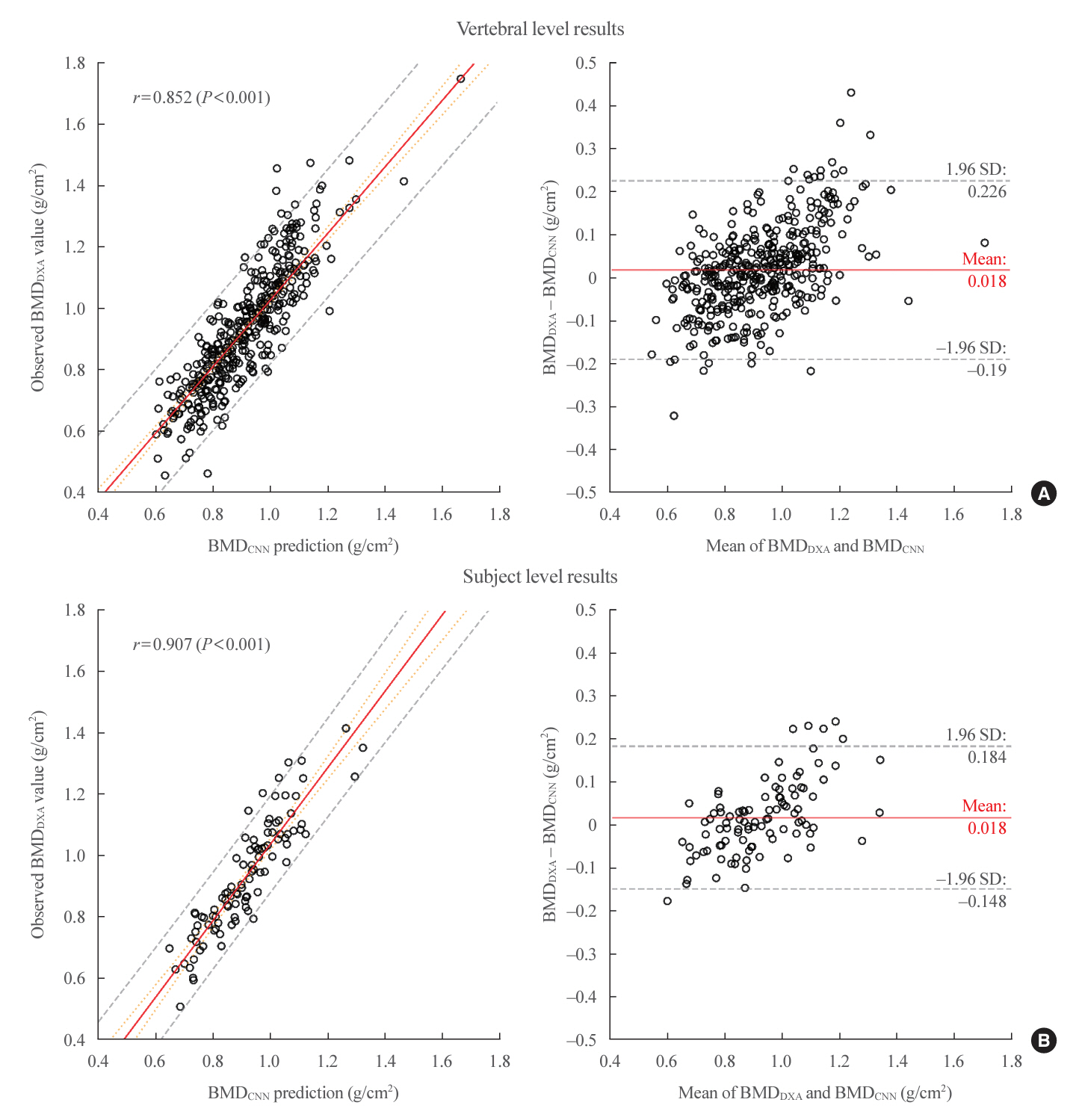
Automated vertebra-level DXA measurements showed a mean absolute error (MAE) of 0.079, Pearson’s r of 0.852 (P<0.001), and R2 of 0.714. Subject-level predictions on the held-out test set had a MAE of 0.066, Pearson’s r of 0.907 (P<0.001), and R2 of 0.781.
Conclusion
CT scans collected during routine examinations without bone densitometry calibration can be used to generate DXA BMD predictions.
Keyword
Figure
Reference
-
1. Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014; 29:2520–6.
Article2. Zeng Q, Li N, Wang Q, Feng J, Sun D, Zhang Q, et al. The prevalence of osteoporosis in China, a nationwide, multicenter DXA survey. J Bone Miner Res. 2019; 34:1789–97.
Article3. Dyer SM, Crotty M, Fairhall N, Magaziner J, Beaupre LA, Cameron ID, et al. A critical review of the long-term disability outcomes following hip fracture. BMC Geriatr. 2016; 16:158.
Article4. Alarkawi D, Bliuc D, Tran T, Ahmed LA, Emaus N, Bjornerem A, et al. Impact of osteoporotic fracture type and subsequent fracture on mortality: the Tromsø Study. Osteoporos Int. 2020; 31:119–30.
Article5. Haentjens P, Magaziner J, Colon-Emeric CS, Vanderschueren D, Milisen K, Velkeniers B, et al. Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med. 2010; 152:380–90.
Article6. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009; 302:1573–9.
Article7. Miller PD. Underdiagnosis and undertreatment of osteoporosis: the battle to be won. J Clin Endocrinol Metab. 2016; 101:852–9.8. Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int. 1994; 4:368–81.9. US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018; 319:2521–31.10. Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014; 25:2359–81.
Article11. Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011; 377:1276–87.
Article12. Buckens CF, Dijkhuis G, de Keizer B, Verhaar HJ, de Jong PA. Opportunistic screening for osteoporosis on routine computed tomography?: an external validation study. Eur Radiol. 2015; 25:2074–9.
Article13. Jang S, Graffy PM, Ziemlewicz TJ, Lee SJ, Summers RM, Pickhardt PJ. Opportunistic osteoporosis screening at routine abdominal and thoracic CT: normative L1 trabecular attenuation values in more than 20,000 adults. Radiology. 2019; 291:360–7.
Article14. Dagan N, Elnekave E, Barda N, Bregman-Amitai O, Bar A, Orlovsky M, et al. Automated opportunistic osteoporotic fracture risk assessment using computed tomography scans to aid in FRAX underutilization. Nat Med. 2020; 26:77–82.
Article15. Yasaka K, Akai H, Kunimatsu A, Kiryu S, Abe O. Prediction of bone mineral density from computed tomography: application of deep learning with a convolutional neural network. Eur Radiol. 2020; 30:3549–57.
Article16. Tang C, Zhang W, Li H, Li L, Li Z, Cai A, et al. CNN-based qualitative detection of bone mineral density via diagnostic CT slices for osteoporosis screening. Osteoporos Int. 2021; 32:971–9.
Article17. Pickhardt PJ, Graffy PM, Zea R, Lee SJ, Liu J, Sandfort V, et al. Automated abdominal CT imaging biomarkers for opportunistic prediction of future major osteoporotic fractures in asymptomatic adults. Radiology. 2020; 297:64–72.
Article18. Fang Y, Li W, Chen X, Chen K, Kang H, Yu P, et al. Opportunistic osteoporosis screening in multi-detector CT images using deep convolutional neural networks. Eur Radiol. 2021; 31:1831–42.
Article19. Liu S, Gonzalez J, Zulueta J, de Torres JP, Yankelevitz DF, Henschke CI, et al. Fully automated bone mineral density assessment from low-dose chest CT. In : Petrick N, Mori K, editors. Proceedings Volume 10575, SPIE Medical Imaging 2018: Computer-Aided Diagnosis; 2018 Feb 10-15; Houston, TX. Bellingham: International Society for Optics and Photonics;2018. Available from: https://doi.org/10.1117/12.2293838.
Article20. Krishnaraj A, Barrett S, Bregman-Amitai O, Cohen-Sfady M, Bar A, Chettrit D, et al. Simulating dual-energy X-ray absorptiometry in CT using deep-learning segmentation cascade. J Am Coll Radiol. 2019; 16:1473–9.
Article21. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004; 363:157–63.22. Belharbi S, Chatelain C, Herault R, Adam S, Thureau S, Chastan M, et al. Spotting L3 slice in CT scans using deep convolutional network and transfer learning. Comput Biol Med. 2017; 87:95–103.
Article23. Kanavati F, Islam S, Aboagye EO, Rockall A. Automatic L3 slice detection in 3D CT images using fully-convolutional networks. arXiv [Preprint]. 2018; Nov. 22. . https://doi.org/10.48550/arXiv.1811.09244.
Article24. Simonyan K, Zisserman A. Very deep convolutional networks for large-scale image recognition. arXiv [Preprint]. 2015; Apr. 10. https://doi.org/10.48550/arXiv.1409.1556.
Article25. Yu EW, Thomas BJ, Brown JK, Finkelstein JS. Simulated increases in body fat and errors in bone mineral density measurements by DXA and QCT. J Bone Miner Res. 2012; 27:119–24.
Article26. Navab N, Hornegger J, Wells WM, Frangi A. Medical Image Computing and Computer-Assisted Intervention: MICCAI 2015. Lecture notes in computer science. Vol. 9351. Cham: Springer;2015. Chapter, U-net: convolutional networks for biomedical image segmentation. 234–41. [cited 2024 Mar 16]. Available from: https://doi.org/10.1007/978-3-319-24574-4_28.
Article27. Kim B, Ye JC. Mumford-Shah loss functional for image segmentation with deep learning. IEEE Trans Image Process. 2020; 29:1856–66.
Article28. Huang G, Liu Z, Van Der Maaten L, Weinberger KQ. Densely connected convolutional networks. In : Proceedings of the 2017 IEEE conference on computer vision and pattern recognition (CVPR); 2017 Jul 21-26; Honolulu, HI. Washington DC: IEEE Computer Society;2017. p. 2261–9. Available from: https://doi.org/10.1109/CVPR.2017.243.
Article29. Paszke A, Gross S, Massa F, Lerer A, Bradbury J, Chanan G, et al. Pytorch: an imperative style, high-performance deep learning library. Adv Neural Inf Process Syst. 2019; 32:1–12.30. Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, et al. Scikit-learn: machine learning in Python. J Mach Learn Res. 2011; 12:2825–30.31. Virtanen P, Gommers R, Oliphant TE, Haberland M, Reddy T, Cournapeau D, et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat Methods. 2020; 17:261–72.32. Altman DG, Bland JM. Measurement in medicine: the analysis of method comparison studies. J R Stat Soc Series D Stat. 1983; 32:307–17.
Article33. Pickhardt PJ, Lee SJ, Liu J, Yao J, Lay N, Graffy PM, et al. Population-based opportunistic osteoporosis screening: validation of a fully automated CT tool for assessing longitudinal BMD changes. Br J Radiol. 2019; 92:20180726.
Article34. Liu S, Xie Y, Reeves AP. Individual bone structure segmentation and labeling from low-dose chest CT. In : Armato SG, Petrick NA, editors. Proceedings Volume 10134, Medical Imaging 2017: Computer-Aided Diagnosis; 2017 Feb 11-16; Orlando. FL. Bellingham: International Society for Optics and Photonics;2017. Available from: https://doi.org/10.1117/12.2254162.
Article35. Summers RM, Baecher N, Yao J, Liu J, Pickhardt PJ, Choi JR, et al. Feasibility of simultaneous computed tomographic colonography and fully automated bone mineral densitometry in a single examination. J Comput Assist Tomogr. 2011; 35:212–6.
Article36. Nam HS, Kweon SS, Choi JS, Zmuda JM, Leung PC, Lui LY, et al. Racial/ethnic differences in bone mineral density among older women. J Bone Miner Metab. 2013; 31:190–8.
Article37. Jancuska JM, Spivak JM, Bendo JA. A review of symptomatic lumbosacral transitional vertebrae: Bertolotti’s syndrome. Int J Spine Surg. 2015; 9:42.
Article38. Carrino JA, Campbell PD Jr, Lin DC, Morrison WB, Schweitzer ME, Flanders AE, et al. Effect of spinal segment variants on numbering vertebral levels at lumbar MR imaging. Radiology. 2011; 259:196–202.
Article39. Kwaan MR, Studdert DM, Zinner MJ, Gawande AA. Incidence, patterns, and prevention of wrong-site surgery. Arch Surg. 2006; 141:353–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Support vector machines are superior to principal components analysis for selecting the optimal bones’ CT attenuations for opportunistic screening for osteoporosis using CT scans of the foot or ankle
- Diagnosis of Scoliosis Using Chest Radiographs with a Semi-Supervised Generative Adversarial Network
- Artificial Intelligence Combined With Chest Radiography: New Hope for the Opportunistic Screening for Osteoporosis
- Response to “Artificial Intelligence Combined With Chest Radiography: New Hope for the Opportunistic Screening for Osteoporosis”
- CT Anatomy of the Diaphragm: Changes in End Inspiration and End Expiration