Korean Circ J.
2024 Jun;54(6):339-350. 10.4070/kcj.2024.0023.
Efficacy and Safety of Sirolimus-Eluting Stent With Biodegradable Polymer Ultimaster™ in Unselected Korean Population: A Multicenter, Prospective, Observational Study From Korean Multicenter Ultimaster Registry
- Affiliations
-
- 1Cardiovascular Center, Korea University Guro Hospital, Seoul, Korea
- 2Cardiovascular Research Institute, Korea University, Seoul, Korea
- 3Division of Cardiology, Department of Internal Medicine, Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul, Korea
- 4Division of Cardiology, Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Incheon, Korea
- 5Division of Cardiology, Department of Internal Medicine, Inha University College of Medicine, Incheon, Korea
- 6Department of Cardiology, Hallym Hospital, Incheon, Korea
- 7Department of Cardiology, Na-Eun Hospital, Incheon, Korea
- 8Division of Cardiology, Department of Internal Medicine, Sejong General Hospital, Bucheon, Korea
- 9Division of Cardiology, Department of Internal Medicine, Kangwon National University School of Medicine, Chuncheon, Korea
- 10Division of Cardiology, Department of Internal Medicine, Eulji University Hospital, Daejeon, Korea
- 11Division of Cardiology, Department of Internal Medicine, Medical Research Institute, Pusan National University Hospital, Busan, Korea
- 12Division of Cardiology, Department of Internal Medicine, Nowon Eulji Medical Center, Eulji University, School of Medicine, Seoul, Korea
- 13Division of Cardiology, Department of Internal Medicine, School of Medicine, Soonchunhyang University Gumi Hospital, Gumi, Korea
- KMID: 2556532
- DOI: http://doi.org/10.4070/kcj.2024.0023
Abstract
- Background and Objectives
Ultimaster™, a third-generation sirolimus-eluting stent using biodegradable polymer, has been introduced to overcome long term adverse vascular events, such as restenosis or stent thrombosis. In the present study, we aimed to evaluate the 12-month clinical outcomes of Ultimaster™ stents in Korean patients with coronary artery disease.
Methods
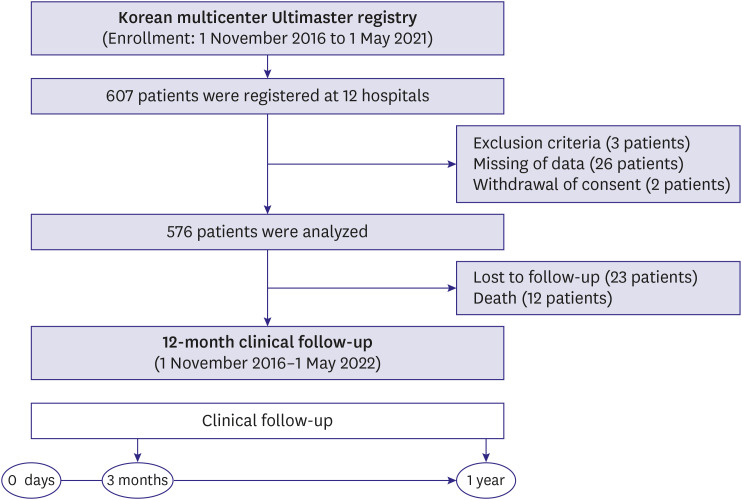
This study is a multicenter, prospective, observational registry across 12 hospitals. To reflect real-world clinical evidence, non-selective subtypes of patients and lesions were included in this study. The study end point was target lesion failure (TLF) (the composite of cardiac death, target vessel myocardial infarction [MI], and target lesion revascularization [TLR]) at 12-month clinical follow up.
Results
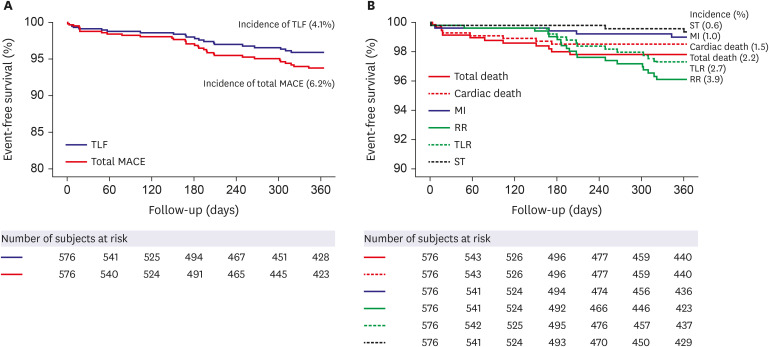
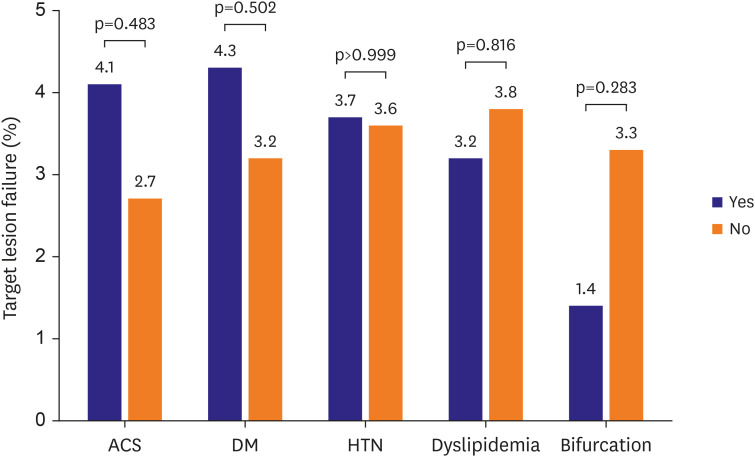
A total of 576 patients were enrolled between November 2016 and May 2021. Most of the patients were male (76.5%), with a mean age of 66.0±11.2 years. Among the included patients, 40.1% had diabetes mellitus (DM) and 67.9% had acute coronary syndrome (ACS). At 12 months, the incidence of TLF was 4.1%. The incidence of cardiac death was 1.5%, MI was 1.0%, TLR was 2.7%, and stent thrombosis was 0.6%. In subgroup analysis based on the presence of ACS, DM, hypertension, dyslipidemia, or bifurcation, there were no major differences in the incidence of the primary endpoint.
Conclusions
The present registry shows that Ultimaster™ stent is safe and effective for routine real-world clinical practice in non-selective Korean patients, having a low rate of adverse events at least up to 12 months.
Figure
Reference
-
1. Morice MC, Serruys PW, Sousa JE, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002; 346:1773–1780. PMID: 12050336.2. Stone GW, Ellis SG, Cox DA, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004; 350:221–231. PMID: 14724301.3. Stettler C, Wandel S, Allemann S, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007; 370:937–948. PMID: 17869634.4. Nakazawa G, Finn AV, Vorpahl M, Ladich ER, Kolodgie FD, Virmani R. Coronary responses and differential mechanisms of late stent thrombosis attributed to first-generation sirolimus- and paclitaxel-eluting stents. J Am Coll Cardiol. 2011; 57:390–398. PMID: 21251578.5. Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006; 48:193–202. PMID: 16814667.6. Serruys PW, Farooq V, Kalesan B, et al. Improved safety and reduction in stent thrombosis associated with biodegradable polymer-based biolimus-eluting stents versus durable polymer-based sirolimus-eluting stents in patients with coronary artery disease: final 5-year report of the LEADERS (Limus Eluted From A Durable Versus ERodable Stent Coating) randomized, noninferiority trial. JACC Cardiovasc Interv. 2013; 6:777–789. PMID: 23968698.7. Barbato E, Salinger-Martinovic S, Sagic D, et al. A first-in-man clinical evaluation of Ultimaster, a new drug-eluting coronary stent system: CENTURY study. EuroIntervention. 2015; 11:541–548. PMID: 25136883.8. Saito S, Valdes-Chavarri M, Richardt G, et al. A randomized, prospective, intercontinental evaluation of a bioresorbable polymer sirolimus-eluting coronary stent system: the CENTURY II (Clinical Evaluation of New Terumo Drug-Eluting Coronary Stent System in the Treatment of Patients with Coronary Artery Disease) trial. Eur Heart J. 2014; 35:2021–2031. PMID: 24847155.9. Godino C, Beneduce A, Ferrante G, et al. One-year clinical outcome of biodegradable polymer sirolimus-eluting stent in all-comers population. Insight from the ULISSE registry (ULtimaster Italian multicenter all comerS Stent rEgistry). Int J Cardiol. 2018; 260:36–41. PMID: 29622449.10. Valdes-Chavarri M, Kedev S, Neskovic AN, et al. Randomised evaluation of a novel biodegradable polymer-based sirolimus-eluting stent in ST-segment elevation myocardial infarction: the MASTER study. EuroIntervention. 2019; 14:e1836–e1842. PMID: 29957593.11. Codner P, Saada M, Sakhov O, et al. Proximal left anterior descending artery treatment using a bioresorbable polymer coating sirolimus-eluting stent: real-world outcomes from the multicenter prospective e-Ultimaster registry. J Am Heart Assoc. 2019; 8:e013786. PMID: 31787055.12. Tadano Y, Kotani JI, Kashima Y, et al. Predictors of clinical outcomes after coronary implantation of bioresorbable polymer sirolimus-eluting Ultimaster stents in all-comers: a report of 1,727 cases. Catheter Cardiovasc Interv. 2019; 94:91–97. PMID: 30636371.13. Iñiguez A, Chevalier B, Richardt G, et al. Comparison of long-term clinical outcomes in multivessel coronary artery disease patients treated either with bioresoarbable polymer sirolimus-eluting stent or permanent polymer everolimus-eluting stent: 5-year results of the CENTURY II randomized clinical trial. Catheter Cardiovasc Interv. 2020; 95:175–184. PMID: 31033154.14. Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation. 2007; 116:2634–2653. PMID: 17951284.15. Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007; 115:2344–2351. PMID: 17470709.16. Medina A, Suárez de Lezo J, Pan M. A new classification of coronary bifurcation lesions. Rev Esp Cardiol. 2006; 59:183. PMID: 16540043.17. Thomas M, Hildick-Smith D, Louvard Y, et al. Percutaneous coronary intervention for bifurcation disease. A consensus view from the first meeting of the European Bifurcation Club. EuroIntervention. 2006; 2:149–153. PMID: 19755253.18. Park TK, Park YH, Song YB, et al. Long-term clinical outcomes of true and non-true bifurcation lesions according to medina classification- results from the COBIS (COronary BIfurcation Stent) II registry. Circ J. 2015; 79:1954–1962. PMID: 26134457.19. Ellis SG, Vandormael MG, Cowley MJ, et al. Coronary morphologic and clinical determinants of procedural outcome with angioplasty for multivessel coronary disease. Implications for patient selection. Multivessel Angioplasty Prognosis Study Group. Circulation. 1990; 82:1193–1202. PMID: 2401060.20. Zhang J. Stent thrombosis in patients with coronary artery disease treated with biodegradable polymer drug-eluting stents: an update meta-analysis. Int Heart J. 2014; 55:213–218. PMID: 24806382.21. Lüscher TF, Steffel J, Eberli FR, et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation. 2007; 115:1051–1058. PMID: 17325255.22. Byrne RA, Iijima R, Mehilli J, et al. Durability of antirestenotic efficacy in drug-eluting stents with and without permanent polymer. JACC Cardiovasc Interv. 2009; 2:291–299. PMID: 19463439.23. Stefanini GG, Byrne RA, Serruys PW, et al. Biodegradable polymer drug-eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: a pooled analysis of individual patient data from the ISAR-TEST 3, ISAR-TEST 4, and LEADERS randomized trials. Eur Heart J. 2012; 33:1214–1222. PMID: 22447805.24. Torii S, Jinnouchi H, Sakamoto A, et al. Drug-eluting coronary stents: insights from preclinical and pathology studies. Nat Rev Cardiol. 2020; 17:37–51. PMID: 31346257.25. Pendyala LK, Matsumoto D, Shinke T, et al. Nobori stent shows less vascular inflammation and early recovery of endothelial function compared with Cypher stent. JACC Cardiovasc Interv. 2012; 5:436–444. PMID: 22516402.26. Iantorno M, Lipinski MJ, Garcia-Garcia HM, et al. Meta-analysis of the impact of strut thickness on outcomes in patients with drug-eluting stents in a coronary artery. Am J Cardiol. 2018; 122:1652–1660. PMID: 30292330.27. Kandzari DE, Mauri L, Koolen JJ, et al. Ultrathin, bioresorbable polymer sirolimus-eluting stents versus thin, durable polymer everolimus-eluting stents in patients undergoing coronary revascularisation (BIOFLOW V): a randomised trial. Lancet. 2017; 390:1843–1852. PMID: 28851504.28. Pilgrim T, Heg D, Roffi M, et al. Ultrathin strut biodegradable polymer sirolimus-eluting stent versus durable polymer everolimus-eluting stent for percutaneous coronary revascularisation (BIOSCIENCE): a randomised, single-blind, non-inferiority trial. Lancet. 2014; 384:2111–2122. PMID: 25189359.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Very Late Stent Thrombosis Related to Fracture of a Sirolimus-Eluting Stent
- Safety and Efficacy of a New Ultrathin Sirolimus-Eluting Stent with Abluminal Biodegradable Polymer in Real-World Practice
- Coronary Stent Thrombosis: Current Insights into New Drug-Eluting Stent Designs
- A Case of Late Recurrent Vasospasm After Sirolimus-Eluting Stent Implantation
- A Case of Stent Thrombosis Occurred at 5 Years after Sirolimus-Eluting Stent Implantation