Int J Thyroidol.
2024 May;17(1):168-181. 10.11106/ijt.2024.17.1.168.
Korean Thyroid Association Guidelines on the Management of Differentiated Thyroid Cancers; Part III. Management of Advanced Differentiated Thyroid Cancers - Chapter 4. Systemic Therapy for Progressive Radioiodine-Refractory Differentiated Thyroid Cancer 2024
- Affiliations
-
- 1Department of Internal Medicine, Severance Hospital, Seoul, Korea
- 2Department of Internal Medicine, Chonnam National University Hwasun Hospital, Hwasun, Korea
- 3Department of Internal Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 4Department of Radiology, Gangneung Asan Hospital, Gangneung, Korea
- 5Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- 6Department of Internal Medicine, Seoul National University Boramae Medical Center, Seoul, Korea
- 7Department of Internal Medicine, National Cancer Center, Goyang, Korea
- 8Department of Internal Medicine, Seoul St. Mary’s Hospital, Seoul, Korea
- 9Department of Internal Medicine, Chung-Ang University Hospital, Seoul, Korea
- 10Department of Internal Medicine, Asan Medical Center, Seoul, Korea
- KMID: 2556513
- DOI: http://doi.org/10.11106/ijt.2024.17.1.168
Abstract
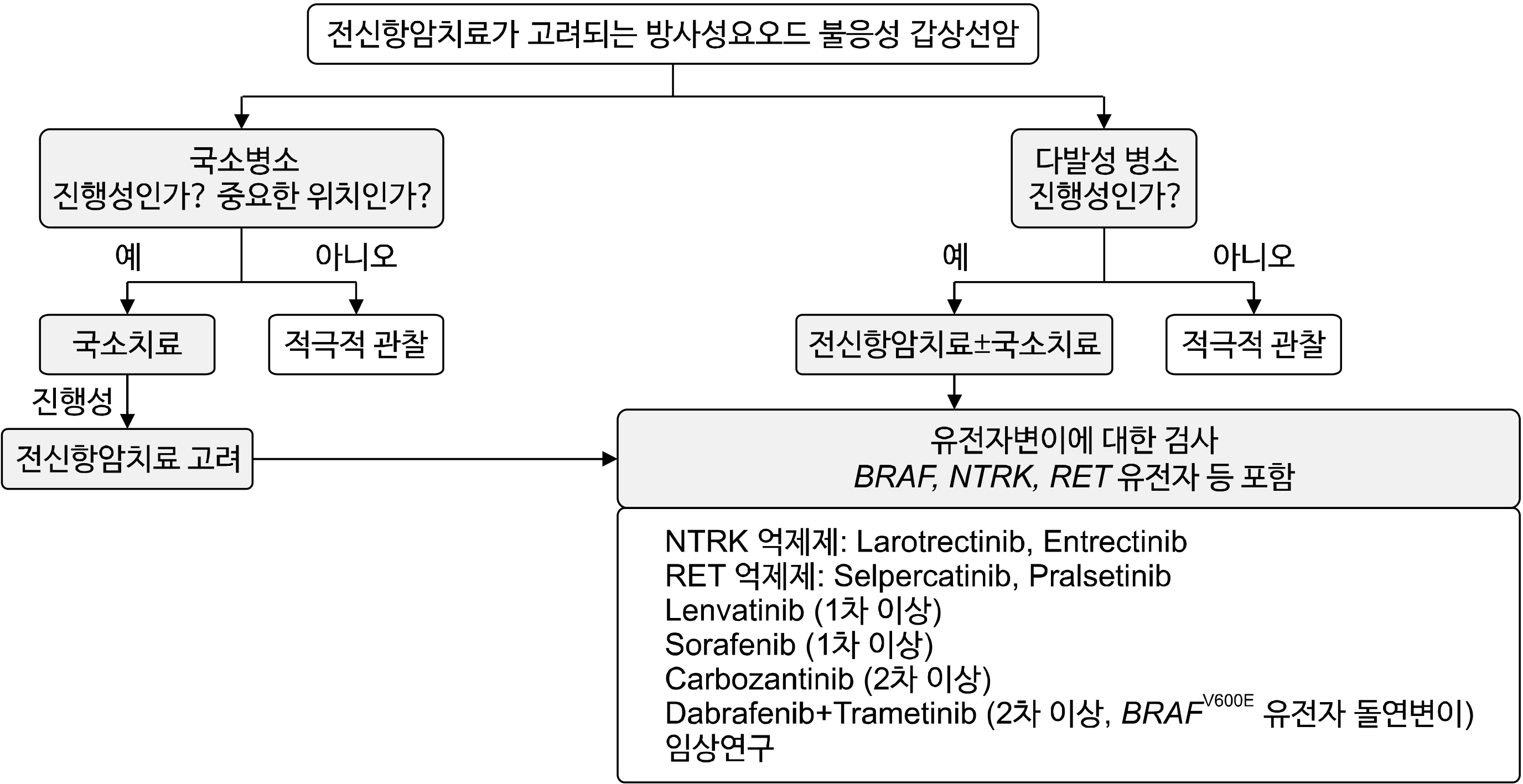
- The primary treatment for differentiated thyroid cancer (DTC) with distant metastasis is high-dose radioactive iodine (RAI) therapy, which can have various effects depending on the iodine uptake of thyroid cancer cells. The iodine uptake of metastatic lesions decreases over time, and approximately 40-70% of patients eventually develop RAI refractory disease. Although the prognosis of patients with RAI refractory DTC is very poor, clinical outcomes vary depending on the location and progression of metastatic lesions. Therefore, it is crucial to determine which patients should receive active systemic therapy with tyrosine kinase inhibitor (TKI) and how to apply local treatment before or during systemic therapy. This guideline covers the definition, treatment principles, systemic anticancer agents, and complications of progressive RAI-refractory DTC. RAI refractory DTC is defined as (1) the absence of RAI uptake on whole body scan, (2) presence of RAI uptake in some lesions but not in others, or (3) disease progression despite RAI uptake. Treatment options for RAI refractory DTC include surgery, external beam radiation therapy, locoregional therapies such as high-intensity focused ultrasound ablation, and systemic anticancer therapy. In patients with minimal symptoms and progression, active surveillance without specific treatment may be considered. Systemic treatment should be considered for patients with multiple progressive lesions by RECIST criteria. Furthermore, testing for cancer gene mutations, including BRAF, NTRK, and RET genes, is recommended for personalized therapy. Systemic therapy should be decided based on shared decision-making between the patient and specialist, considering anticipated benefits and risks. Regular assessment of treatment responses and evaluation of adverse events is essential, with dose adjustment based on these assessments. The optimal time of use, clinical approaches for the prevention and control of adverse events, and individualized treatment approaches based on patient characteristics will be of great help in the treatment of patients with RAI-refractory DTC.
Keyword
Figure
Reference
-
References
1. Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, et al. 2006; Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 91(8):2892–9. DOI: 10.1210/jc.2005-2838. PMID: 16684830.
Article2. Cho SW, Choi HS, Yeom GJ, Lim JA, Moon JH, Park DJ, et al. 2014; Long-term prognosis of differentiated thyroid cancer with lung metastasis in Korea and its prognostic factors. Thyroid. 24(2):277–86. DOI: 10.1089/thy.2012.0654. PMID: 23758653. PMCID: PMC3926138.
Article3. Kim M, Kim WG, Park SY, Kwon H, Jeon MJ, Lee JJ, et al. 2017; Initial size of metastatic lesions is best prognostic factor in patients with metastatic differentiated thyroid carcinoma confined to the lung. Thyroid. 27(1):49–58. DOI: 10.1089/thy.2016.0347. PMID: 27750021.
Article4. Feine U, Lietzenmayer R, Hanke JP, Held J, Wohrle H, Muller-Schauenburg W. 1996; Fluorine-18-FDG and iodine-131-iodide uptake in thyroid cancer. J Nucl Med. 37(9):1468–72.5. Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. 2014; Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 384(9940):319–28. DOI: 10.1016/S0140-6736(14)60421-9. PMID: 24768112.
Article6. Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. 2015; Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 372(7):621–30. DOI: 10.1056/NEJMoa1406470. PMID: 25671254.
Article7. Brose MS, Robinson B, Sherman SI, Krajewska J, Lin CC, Vaisman F, et al. 2021; Cabozantinib for radioiodine-refractory differentiated thyroid cancer (COSMIC-311): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 22(8):1126–38. DOI: 10.1016/S1470-2045(21)00332-6. PMID: 34237250.
Article8. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2016; 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 26(1):1–133. DOI: 10.1089/thy.2015.0020. PMID: 26462967. PMCID: PMC4739132.
Article9. Fugazzola L, Elisei R, Fuhrer D, Jarzab B, Leboulleux S, Newbold K, et al. 2019; 2019 European Thyroid Association guidelines for the treatment and follow-up of advanced radioiodine-refractory thyroid cancer. Eur Thyroid J. 8(5):227–45. DOI: 10.1159/000502229. PMID: 31768334. PMCID: PMC6873012.
Article10. Tuttle RM, Ahuja S, Avram AM, Bernet VJ, Bourguet P, Daniels GH, et al. 2019; Controversies, consensus, and collaboration in the use of (131)I therapy in differentiated thyroid cancer: a joint statement from the American Thyroid Association, the European Association of Nuclear Medicine, the Society of Nuclear Medicine and Molecular Imaging, and the European Thyroid Association. Thyroid. 29(4):461–70. DOI: 10.1089/thy.2018.0597. PMID: 30900516.
Article11. Cabanillas ME, Terris DJ, Sabra MM. 2017; Information for clinicians: approach to the patient with progressive radioiodine-refractory thyroid cancer-when to use systemic therapy. Thyroid. 27(8):987–93. DOI: 10.1089/thy.2016.0578. PMID: 28635520.
Article12. Van Nostrand D. 2018; Radioiodine refractory differentiated thyroid cancer: time to update the classifications. Thyroid. 28(9):1083–93. DOI: 10.1089/thy.2018.0048. PMID: 30105931.
Article13. Sabra MM, Grewal RK, Tala H, Larson SM, Tuttle RM. 2012; Clinical outcomes following empiric radioiodine therapy in patients with structurally identifiable metastatic follicular cell-derived thyroid carcinoma with negative diagnostic but positive post-therapy 131I whole-body scans. Thyroid. 22(9):877–83. DOI: 10.1089/thy.2011.0429. PMID: 22827641.
Article14. Leboulleux S, El Bez I, Borget I, Elleuch M, Deandreis D, Al Ghuzlan A, et al. 2012; Postradioiodine treatment whole-body scan in the era of 18-fluorodeoxyglucose positron emission tomography for differentiated thyroid carcinoma with elevated serum thyroglobulin levels. Thyroid. 22(8):832–8. DOI: 10.1089/thy.2012.0081. PMID: 22853728.
Article15. Schwartz LH, Seymour L, Litiere S, Ford R, Gwyther S, Mandrekar S, et al. 2016; RECIST 1.1 - Standardisation and disease-specific adaptations: perspectives from the RECIST Working Group. Eur J Cancer. 62:138–45. DOI: 10.1016/j.ejca.2016.03.082. PMID: 27237360. PMCID: PMC5737786.
Article16. Filetti S, Durante C, Hartl D, Leboulleux S, Locati LD, Newbold K, et al. 2019; Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 30(12):1856–83. DOI: 10.1093/annonc/mdz400. PMID: 31549998.
Article17. Aashiq M, Silverman DA, Na'ara S, Takahashi H, Amit M. 2019; Radioiodine-refractory thyroid cancer: molecular basis of redifferentiation therapies, management, and novel therapies. Cancers (Basel). 11(9):1382. DOI: 10.3390/cancers11091382. PMID: 31533238. PMCID: PMC6770909.
Article18. Shen X, Liu R, Xing M. 2017; A six-genotype genetic prognostic model for papillary thyroid cancer. Endocr Relat Cancer. 24(1):41–52. DOI: 10.1530/ERC-16-0402. PMID: 27875244. PMCID: PMC5132178.
Article19. Ho AL, Dedecjus M, Wirth LJ, Tuttle RM, Inabnet WB 3rd, Tennvall J, et al. 2022; Selumetinib plus adjuvant radioactive iodine in patients with high-risk differentiated thyroid cancer: a phase III, randomized, placebo-controlled trial (ASTRA). J Clin Oncol. 40(17):1870–8. DOI: 10.1200/JCO.21.00714. PMID: 35192411. PMCID: PMC9851689.
Article20. Rothenberg SM, McFadden DG, Palmer EL, Daniels GH, Wirth LJ. 2015; Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib. Clin Cancer Res. 21(5):1028–35. DOI: 10.1158/1078-0432.CCR-14-2915. PMID: 25549723.21. Leboulleux S, Do Cao C, Zerdoud S, Attard M, Bournaud C, Lacroix L, et al. 2023; A phase II redifferentiation trial with dabrafenib-trametinib and 131I in metastatic radioactive iodine refractory BRAF p.V600E-mutated differentiated thyroid cancer. Clin Cancer Res. 29(13):2401–9. DOI: 10.1158/1078-0432.CCR-23-0046. PMID: 37074727.22. Lee YA, Lee H, Im SW, Song YS, Oh DY, Kang HJ, et al. 2021; NTRK and RET fusion-directed therapy in pediatric thyroid cancer yields a tumor response and radioiodine uptake. J Clin Invest. 131(18):e144847. DOI: 10.1172/JCI144847. PMID: 34237031. PMCID: PMC8439610.23. Ho AL, Grewal RK, Leboeuf R, Sherman EJ, Pfister DG, Deandreis D, et al. 2013; Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. 368(7):623–32. DOI: 10.1056/NEJMoa1209288. PMID: 23406027. PMCID: PMC3615415.
Article24. Filetti S, Durante C, Hartl DM, Leboulleux S, Locati LD, Newbold K, et al. 2022; ESMO clinical practice guideline update on the use of systemic therapy in advanced thyroid cancer. Ann Oncol. 33(7):674–84. DOI: 10.1016/j.annonc.2022.04.009. PMID: 35491008.
Article25. Haddad RI, Bischoff L, Salgado SA, Applewhite M, Bernet V, Blomain E, et al. NCCN clinical practice guidelines in oncology (NCCN Guidelines®): Thyroid carcinoma. version 1.2024. Available from: URL: https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf.26. Zheng X, Xu Z, Ji Q, Ge M, Shi F, Qin J, et al. 2021; A randomized, phase III study of lenvatinib in Chinese patients with radioiodine-refractory differentiated thyroid cancer. Clin Cancer Res. 27(20):5502–9. DOI: 10.1158/1078-0432.CCR-21-0761. PMID: 34326132. PMCID: PMC9401493.
Article27. Cancer Genome Atlas Research Network. 2014; Integrated genomic characterization of papillary thyroid carcinoma. Cell. 159(3):676–90. DOI: 10.1016/j.cell.2014.09.050. PMID: 25417114. PMCID: PMC4243044.28. Drilon A, Hu ZI, Lai GGY, Tan DSW. 2018; Targeting RET-driven cancers: lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol. 15(3):151–67. DOI: 10.1038/nrclinonc.2017.175. PMID: 29134959. PMCID: PMC7938338.
Article29. Subbiah V, Cote GJ. 2020; Advances in targeting RET-dependent cancers. Cancer Discov. 10(4):498–505. DOI: 10.1158/2159-8290.CD-19-1116. PMID: 32094155. PMCID: PMC7125013.
Article30. Hechtman JF. 2022; NTRK insights: best practices for pathologists. Mod Pathol. 35(3):298–305. DOI: 10.1038/s41379-021-00913-8. PMID: 34531526. PMCID: PMC8860742.
Article31. Yakushina VD, Lerner LV, Lavrov AV. 2018; Gene fusions in thyroid cancer. Thyroid. 28(2):158–67. DOI: 10.1089/thy.2017.0318. PMID: 29281951.
Article32. Lee SE, Lee MS, Jeon YK, Shim HS, Kang J, Kim J, et al. 2023; Interlaboratory comparison study (ring test) of next-generation sequencing-based NTRK fusion detection in South Korea. Cancer Res Treat. 55(1):28–40. DOI: 10.4143/crt.2021.1572. PMID: 35167738. PMCID: PMC9873325.
Article33. Salvatore D, Santoro M, Schlumberger M. 2021; The importance of the RET gene in thyroid cancer and therapeutic implications. Nat Rev Endocrinol. 17(5):296–306. DOI: 10.1038/s41574-021-00470-9. PMID: 33603219.
Article34. Wirth LJ, Sherman E, Robinson B, Solomon B, Kang H, Lorch J, et al. 2020; Efficacy of Selpercatinib in RET-altered thyroid cancers. N Engl J Med. 383(9):825–35. DOI: 10.1056/NEJMoa2005651. PMID: 32846061. PMCID: PMC10777663.
Article35. Subbiah V, Hu MI, Wirth LJ, Schuler M, Mansfield AS, Curigliano G, et al. 2021; Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): a multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol. 9(8):491–501. DOI: 10.1016/S2213-8587(21)00120-0. PMID: 34118198.
Article36. Subbiah V, Hu MI, Mansfield AS, Taylor MH, Schuler M, Zhu VW, et al. 2024; Pralsetinib in patients with advanced/metastatic rearranged during transfection (RET)-altered thyroid cancer: updated efficacy and safety data from the ARROW Study. Thyroid. 34(1):26–40. DOI: 10.1089/thy.2023.0363. PMID: 38009200.
Article37. Laetsch TW, DuBois SG, Mascarenhas L, Turpin B, Federman N, Albert CM, et al. 2018; Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol. 19(5):705–14. DOI: 10.1016/S1470-2045(18)30119-0. PMID: 29606586.
Article38. Waguespack SG, Drilon A, Lin JJ, Brose MS, McDermott R, Almubarak M, et al. 2022; Efficacy and safety of larotrectinib in patients with TRK fusion-positive thyroid carcinoma. Eur J Endocrinol. 186(6):631–43. DOI: 10.1530/EJE-21-1259. PMID: 35333737. PMCID: PMC9066591.
Article39. Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, et al. 2018; Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 378(8):731–9. DOI: 10.1056/NEJMoa1714448. PMID: 29466156. PMCID: PMC5857389.
Article40. Demetri GD, De Braud F, Drilon A, Siena S, Patel MR, Cho BC, et al. 2022; Updated integrated analysis of the efficacy and safety of entrectinib in patients with NTRK fusion-positive solid tumors. Clin Cancer Res. 28(7):1302–12. DOI: 10.1158/1078-0432.CCR-21-3597. PMID: 35144967. PMCID: PMC9365368.41. Busaidy NL, Konda B, Wei L, Wirth LJ, Devine C, Daniels GA, et al. 2022; Dabrafenib versus dabrafenib + trametinib in BRAF-mutated radioactive iodine refractory differentiated thyroid cancer: results of a randomized, phase 2, open-label multicenter trial. Thyroid. 32(10):1184–92. DOI: 10.1089/thy.2022.0115. PMID: 35658604. PMCID: PMC9595631.42. Chrisoulidou A, Mandanas S, Margaritidou E, Mathiopoulou L, Boudina M, Georgopoulos K, et al. 2015; Treatment compliance and severe adverse events limit the use of tyrosine kinase inhibitors in refractory thyroid cancer. Onco Targets Ther. 8:2435–42. DOI: 10.2147/OTT.S86322. PMID: 26366098. PMCID: PMC4562763.43. Blevins DP, Dadu R, Hu M, Baik C, Balachandran D, Ross W, et al. 2014; Aerodigestive fistula formation as a rare side effect of antiangiogenic tyrosine kinase inhibitor therapy for thyroid cancer. Thyroid. 24(5):918–22. DOI: 10.1089/thy.2012.0598. PMID: 24635127. PMCID: PMC4026371.
Article44. Kim M, Jin M, Jeon MJ, Kim EY, Shin DY, Lim DJ, et al. 2023; Lenvatinib compared with sorafenib as a first-line treatment for radioactive iodine-refractory, progressive, differentiated thyroid carcinoma: real-world outcomes in a multicenter retrospective cohort study. Thyroid. 33(1):91–9. DOI: 10.1089/thy.2022.0054. PMID: 35443825.
Article45. Jeon Y, Park S, Lee SH, Kim TH, Kim SW, Ahn MJ, et al. Combination of dabrafenib and trametinib in patients with metastatic BRAFV600E-mutated thyroid cancer. Cancer Res Treat. 2024; [Online ahead of print]. DOI: 10.4143/crt.2023.1278.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Second Primary Malignancy after Radioiodine Treatment of Thyroid Disease: Current Status
- New Trends in Radioiodine Treatment for the Advanced Differentiated Thyroid Cancer
- Current Status and Future Perspective of the Treatment for Radioiodine Refractory Differentiated Thyroid Cancer
- Recent Advances in Radioiodine Therapy for Thyroid Cancer
- Differentiated Thyroid Cancer and Radioactive Iodine: Past, Present and Future