Int J Thyroidol.
2024 May;17(1):97-110. 10.11106/ijt.2024.17.1.97.
Korean Thyroid Association Guidelines on the Management of Differentiated Thyroid Cancers; Part I. Initial Management of Differentiated Thyroid Cancers - Chapter 6. Radioactive Iodine Treatment after Thyroidectomy 2024
- Affiliations
-
- 1Department of Nuclear Medicine, National Cancer Center, Goyang, Korea
- 2Department of Nuclear Medicine, Chosun University Hospital, Gwangju, Korea
- 3Department of Internal Medicine, Chonnam National University Hwasun Hospital, Hwasun, Korea
- 4Department of Nuclear Medicine, Pusan National University Hospital, Busan, Korea
- 5Department of Internal Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 6Department of Radiology, Gangneung Asan Hospital, Gangneung, Korea
- 7Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- 8Department of Nuclear Medicine, CHA Bundang Medical Center, Seongnam, Korea
- 9Department of Nuclear Medicine, Chungnam National University Sejong Hospital, Sejong, Korea
- 10Department of Internal Medicine, Seoul National University Boramae Medical Center, Seoul, Korea
- 11Department of Nuclear Medicine, Seoul National University Boramae Medical Center, Seoul, Korea
- 12Department of Internal Medicine, National Cancer Center, Goyang, Korea
- 13Department of Internal Medicine, Seoul St. Mary’s Hospital, Seoul, Korea
- 14Department of Internal Medicine, Chung-Ang University Hospital, Seoul, Korea
- 15Department of Nuclear Medicine, Kyungpook National University Hospital, Daegu, Korea
- 16Department of Nuclear Medicine, Kyungpook National University Chilgok Hospital, Daegu, Korea
- KMID: 2556508
- DOI: http://doi.org/10.11106/ijt.2024.17.1.97
Abstract
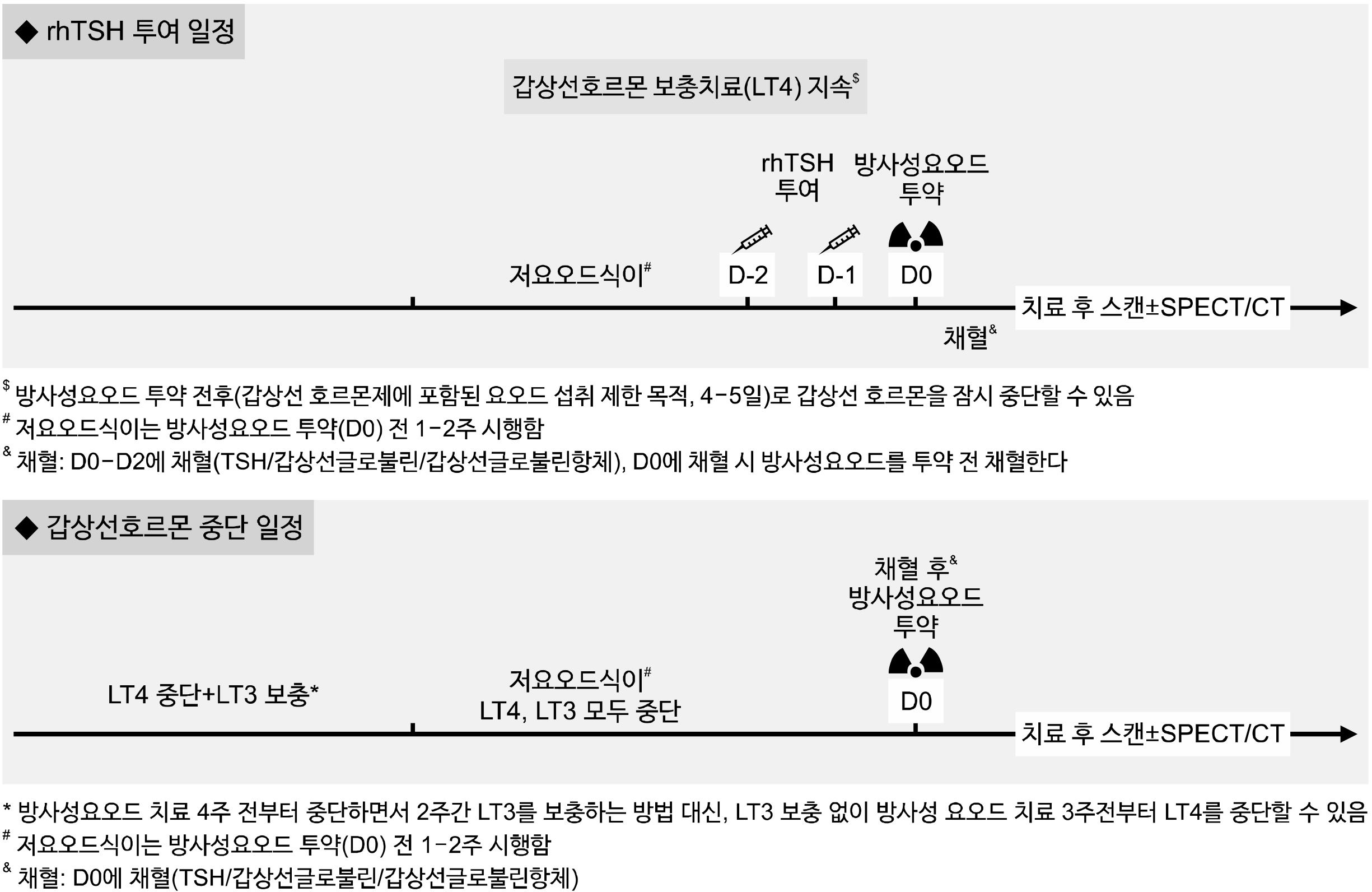
- The initial treatment for differentiated thyroid cancer includes appropriate surgery and radioactive iodine (RAI) therapy, followed by thyroid-stimulating hormone (TSH) suppression therapy as long-term management to prevent recurrence. RAI therapy following thyroidectomy has the three main purposes: remnant ablation, adjuvant therapy, and therapy for known disease. To optimize the goals and targets of RAI therapy, postoperative disease assessment, determination of recurrence risk, and consideration of various individual factors are necessary. The objectives of RAI therapy are determined based on the individual’s recurrence risk, and the administered activity of RAI is then determined according to these treatment objectives. Adequate stimulation of serum TSH is necessary before RAI therapy, and recombinant human TSH is widely used because of its advantage in reducing the risk of exacerbation of comorbidities associated with levothyroxine discontinuation and improving patients’ quality of life. Additionally, reducing iodine intake through appropriate low-iodine diet is necessary. Whole-body scans are conducted to assess the disease status after RAI therapy. If planar whole-body scans are inconclusive, additional single-photon emission computed tomography (SPECT)/CT imaging is recommended. Over the past decade, prospective randomized or retrospective clinical studies on the selection of candidates for RAI therapy, administered activity, methods of TSH stimulation, and advantages of SPECT/CT have been published. Based on these latest clinical research findings and recommendations from relevant overseas medical societies, this clinical practice guideline presents the indications and methods for administering RAI therapy after thyroidectomy.
Keyword
Figure
Reference
-
References
1. Avram AM, Fig LM, Frey KA, Gross MD, Wong KK. 2013; Preablation 131-I scans with SPECT/CT in postoperative thyroid cancer patients: what is the impact on staging? J Clin Endocrinol Metab. 98(3):1163–71. DOI: 10.1210/jc.2012-3630. PMID: 23430789.
Article2. Chen MK, Yasrebi M, Samii J, Staib LH, Doddamane I, Cheng DW. 2012; The utility of I-123 pretherapy scan in I-131 radioiodine therapy for thyroid cancer. Thyroid. 22(3):304–9. DOI: 10.1089/thy.2011.0203. PMID: 22300251.
Article3. Van Nostrand D, Aiken M, Atkins F, Moreau S, Garcia C, Acio E, et al. 2009; The utility of radioiodine scans prior to iodine 131 ablation in patients with well-differentiated thyroid cancer. Thyroid. 19(8):849–55. DOI: 10.1089/thy.2008.0419. PMID: 19281428.
Article4. Lee SW. 2017; SPECT/CT in the treatment of differentiated thyroid cancer. Nucl Med Mol Imaging. 51(4):297–303. DOI: 10.1007/s13139-017-0473-x. PMID: 29242723. PMCID: PMC5721084.
Article5. Song H, Mosci C, Akatsu H, Basina M, Dosiou C, Iagaru A. 2018; Diagnostic 123I whole body scan prior to ablation of thyroid remnant in patients with papillary thyroid cancer: implications for clinical management. Clin Nucl Med. 43(10):705–9. DOI: 10.1097/RLU.0000000000002246.
Article6. de Koster EJ, Sulaiman T, Hamming JF, Schepers A, Snel M, van Velden FHP, et al. 2021; Radioiodine in differentiated thyroid carcinoma: do we need diagnostic pre-ablation iodine-123 scintigraphy to optimize treatment? Diagnostics (Basel). 11(3):553. DOI: 10.3390/diagnostics11030553. PMID: 33808843. PMCID: PMC8003652.
Article7. Gulec SA, Ahuja S, Avram AM, Bernet VJ, Bourguet P, Draganescu C, et al. 2021; A joint statement from the American Thyroid Association, the European Association of Nuclear Medicine, the European Thyroid Association, the Society of Nuclear Medicine and Molecular Imaging on current diagnostic and theranostic approaches in the management of thyroid cancer. Thyroid. 31(7):1009–19. DOI: 10.1089/thy.2020.0826. PMID: 33789450.
Article8. Wong KK, Sisson JC, Koral KF, Frey KA, Avram AM. 2010; Staging of differentiated thyroid carcinoma using diagnostic 131I SPECT/CT. AJR Am J Roentgenol. 195(3):730–6. DOI: 10.2214/AJR.09.3458. PMID: 20729453.9. Chong A, Seo Y, Bang JI, Park S, Kim K, Hong CM, et al. 2024; Clinical implications of adding SPECT/CT to radioiodine whole-body scan in patients with differentiated thyroid cancer: a systematic review and meta-analysis. Clin Nucl Med. 49(3):215–25. DOI: 10.1097/RLU.0000000000004953. PMID: 38048517.
Article10. Avram AM, Esfandiari NH, Wong KK. 2015; Preablation 131-I scans with SPECT/CT contribute to thyroid cancer risk stratification and 131-I therapy planning. J Clin Endocrinol Metab. 100(5):1895–902. DOI: 10.1210/jc.2014-4043. PMID: 25734251.
Article11. Gerard SK, Cavalieri RR. 2002; I-123 diagnostic thyroid tumor whole-body scanning with imaging at 6, 24, and 48 hours. Clin Nucl Med. 27(1):1–8. DOI: 10.1097/00003072-200201000-00001. PMID: 11805475.
Article12. Tuttle RM, Ahuja S, Avram AM, Bernet VJ, Bourguet P, Daniels GH, et al. 2019; Controversies, consensus, and collaboration in the use of (131)I therapy in differentiated thyroid cancer: a joint statement from the American Thyroid Association, the European Association of Nuclear Medicine, the Society of Nuclear Medicine and Molecular Imaging, and the European Thyroid Association. Thyroid. 29(4):461–70. DOI: 10.1089/thy.2018.0597. PMID: 30900516.
Article13. Schvartz C, Bonnetain F, Dabakuyo S, Gauthier M, Cueff A, Fieffe S, et al. 2012; Impact on overall survival of radioactive iodine in low-risk differentiated thyroid cancer patients. J Clin Endocrinol Metab. 97(5):1526–35. DOI: 10.1210/jc.2011-2512. PMID: 22344193.
Article14. Jonklaas J, Sarlis NJ, Litofsky D, Ain KB, Bigos ST, Brierley JD, et al. 2006; Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid. 16(12):1229–42. DOI: 10.1089/thy.2006.16.1229. PMID: 17199433.
Article15. Jonklaas J, Cooper DS, Ain KB, Bigos T, Brierley JD, Haugen BR, et al. 2010; Radioiodine therapy in patients with stage I differentiated thyroid cancer. Thyroid. 20(12):1423–4. DOI: 10.1089/thy.2010.0308. PMID: 21054207.
Article16. Leboulleux S, Bournaud C, Chougnet CN, Zerdoud S, Al Ghuzlan A, Catargi B, et al. 2022; Thyroidectomy without radioiodine in patients with low-risk thyroid cancer. N Engl J Med. 386(10):923–32. DOI: 10.1056/NEJMoa2111953. PMID: 35263518.
Article17. Mallick U, Harmer C, Hackshaw A, Moss L. IoN Trial Management Group. 2012; Iodine or not (IoN) for low-risk differentiated thyroid cancer: the next UK National Cancer Research Network randomised trial following HiLo. Clin Oncol (R Coll Radiol). 24(3):159–61. DOI: 10.1016/j.clon.2012.01.001. PMID: 22316618.
Article18. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2016; 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 26(1):1–133. DOI: 10.1089/thy.2015.0020. PMID: 26462967. PMCID: PMC4739132.
Article19. Hindie E, Giovanella L, Taieb D, Avram AM. 2019; Thyroid cancer recurrence in the HiLo trial. Lancet Diabetes Endocrinol. 7(4):252. DOI: 10.1016/S2213-8587(19)30070-1. PMID: 30902264.
Article20. Verburg FA, Aktolun C, Chiti A, Frangos S, Giovanella L, Hoffmann M, et al. 2016; Why the European Association of Nuclear Medicine has declined to endorse the 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 43(6):1001–5. DOI: 10.1007/s00259-016-3327-3. PMID: 26883666.
Article21. Luster M, Aktolun C, Amendoeira I, Barczynski M, Bible KC, Duntas LH, et al. 2019; European perspective on 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: Proceedings of an Interactive International Symposium. Thyroid. 29(1):7–26. DOI: 10.1089/thy.2017.0129. PMID: 30484394.
Article22. Avram AM, Giovanella L, Greenspan B, Lawson SA, Luster M, Van Nostrand D, et al. 2022; SNMMI procedure standard/EANM practice guideline for nuclear medicine evaluation and therapy of differentiated thyroid cancer: abbreviated version. J Nucl Med. 63(6):15N–35N.23. Petranović Ovčariček P, Kreissl MC, Campenni A, de Keizer B, Tuncel M, Vrachimis A, et al. 2022; SNMMI/EANM practice guideline vs. ETA consensus statement: differences and similarities in approaching differentiated thyroid cancer management-the EANM perspective. Eur J Nucl Med Mol Imaging. 49(12):3959–63. DOI: 10.1007/s00259-022-05935-1. PMID: 35947178.
Article24. Schmidt M, Bartenstein P, Bucerius J, Dietlein M, Drzezga A, Herrmann K, et al. 2022; Individualized treatment of differentiated thyroid cancer: the value of surgery in combination with radioiodine imaging and therapy - a German position paper from Surgery and Nuclear Medicine. Nuklearmedizin. 61(2):87–96. DOI: 10.1055/a-1783-8154. PMID: 35299276.
Article25. Kazaure HS, Roman SA, Sosa JA. 2012; Aggressive variants of papillary thyroid cancer: incidence, characteristics and predictors of survival among 43,738 patients. Ann Surg Oncol. 19(6):1874–80. DOI: 10.1245/s10434-011-2129-x. PMID: 22065195.
Article26. Ruel E, Thomas S, Dinan M, Perkins JM, Roman SA, Sosa JA. 2015; Adjuvant radioactive iodine therapy is associated with improved survival for patients with intermediate-risk papillary thyroid cancer. J Clin Endocrinol Metab. 100(4):1529–36. DOI: 10.1210/jc.2014-4332. PMID: 25642591. PMCID: PMC4399282.
Article27. Sawka AM, Brierley JD, Tsang RW, Thabane L, Rotstein L, Gafni A, et al. 2008; An updated systematic review and commentary examining the effectiveness of radioactive iodine remnant ablation in well-differentiated thyroid cancer. Endocrinol Metab Clin North Am. 37(2):457–80. xDOI: 10.1016/j.ecl.2008.02.007. PMID: 18502337.
Article28. Lamartina L, Durante C, Filetti S, Cooper DS. 2015; Low-risk differentiated thyroid cancer and radioiodine remnant ablation: a systematic review of the literature. J Clin Endocrinol Metab. 100(5):1748–61. DOI: 10.1210/jc.2014-3882. PMID: 25679996.
Article29. Klain M, Nappi C, Zampella E, Cantoni V, Green R, Piscopo L, et al. 2021; Ablation rate after radioactive iodine therapy in patients with differentiated thyroid cancer at intermediate or high risk of recurrence: a systematic review and a meta-analysis. Eur J Nucl Med Mol Imaging. 48(13):4437–44. DOI: 10.1007/s00259-021-05440-x. PMID: 34142215. PMCID: PMC8566414.
Article30. Chow SM, Yau S, Kwan CK, Poon PC, Law SC. 2006; Local and regional control in patients with papillary thyroid carcinoma: specific indications of external radiotherapy and radioactive iodine according to T and N categories in AJCC 6th edition. Endocr Relat Cancer. 13(4):1159–72. DOI: 10.1677/erc.1.01320. PMID: 17158761.
Article31. Lee EK, Lee YJ, Park YJ, Moon JH, Yi KH, Kim KS, et al. 2020; A phase II multi-center, non-randomized, parallel group, non- inferiority study to compare the efficacy of no radioactive iodine remnant ablation to remnant ablation treatment in low- to intermediate-risk of papillary thyroid cancer: the MOREthyroid trial protocol. Endocrinol Metab (Seoul). 35(3):571–7. DOI: 10.3803/EnM.2020.681. PMID: 32981299. PMCID: PMC7520583.32. Pacini F, Fuhrer D, Elisei R, Handkiewicz-Junak D, Leboulleux S, Luster M, et al. 2022; 2022 ETA consensus statement: what are the indications for post-surgical radioiodine therapy in differentiated thyroid cancer? Eur Thyroid J. 11(1):e210046. DOI: 10.1530/ETJ-21-0046. PMID: 34981741. PMCID: PMC9142814.
Article33. Podnos YD, Smith DD, Wagman LD, Ellenhorn JD. 2007; Survival in patients with papillary thyroid cancer is not affected by the use of radioactive isotope. J Surg Oncol. 96(1):3–7. DOI: 10.1002/jso.20656. PMID: 17567872.
Article34. Filetti S, Durante C, Hartl D, Leboulleux S, Locati LD, Newbold K, et al. 2019; Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 30(12):1856–83. DOI: 10.1093/annonc/mdz400. PMID: 31549998.
Article35. Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, et al. 2006; Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 91(8):2892–9. DOI: 10.1210/jc.2005-2838. PMID: 16684830.
Article36. Mallick U, Harmer C, Yap B, Wadsley J, Clarke S, Moss L, et al. 2012; Ablation with low-dose radioiodine and thyrotropin alfa in thyroid cancer. N Engl J Med. 366(18):1674–85. DOI: 10.1056/NEJMoa1109589. PMID: 22551128.
Article37. Schlumberger M, Catargi B, Borget I, Deandreis D, Zerdoud S, Bridji B, et al. 2012; Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N Engl J Med. 366(18):1663–73. DOI: 10.1056/NEJMoa1108586. PMID: 22551127.
Article38. Dehbi HM, Mallick U, Wadsley J, Newbold K, Harmer C, Hackshaw A. 2019; Recurrence after low-dose radioiodine ablation and recombinant human thyroid-stimulating hormone for differentiated thyroid cancer (HiLo): long-term results of an open-label, non-inferiority randomised controlled trial. Lancet Diabetes Endocrinol. 7(1):44–51. DOI: 10.1016/S2213-8587(18)30306-1. PMID: 30501974.
Article39. Schlumberger M, Leboulleux S, Catargi B, Deandreis D, Zerdoud S, Bardet S, et al. 2018; Outcome after ablation in patients with low-risk thyroid cancer (ESTIMABL1): 5-year follow-up results of a randomised, phase 3, equivalence trial. Lancet Diabetes Endocrinol. 6(8):618–26. DOI: 10.1016/S2213-8587(18)30113-X. PMID: 29807824.
Article40. James DL, Ryan EJ, Davey MG, Quinn AJ, Heath DP, Garry SJ, et al. 2021; Radioiodine remnant ablation for differentiated thyroid cancer: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 147(6):544–52. DOI: 10.1001/jamaoto.2021.0288. PMID: 33792650. PMCID: PMC8017484.
Article41. Han JM, Kim WG, Kim TY, Jeon MJ, Ryu JS, Song DE, et al. 2014; Effects of low-dose and high-dose postoperative radioiodine therapy on the clinical outcome in patients with small differentiated thyroid cancer having microscopic extrathyroidal extension. Thyroid. 24(5):820–5. DOI: 10.1089/thy.2013.0362. PMID: 24328997. PMCID: PMC4026370.
Article42. Wang C, Zhao T, Li H, Gao W, Lin Y. 2017; Low activity versus high activity: noninferior response to radioiodine therapy in differentiated patients with extrathyroid extension. Nucl Med Commun. 38(5):366–71. DOI: 10.1097/MNM.0000000000000666. PMID: 28362717.43. Ha S, Oh SW, Kim YK, Koo do H, Jung YH, Yi KH, et al. 2015; Clinical outcome of remnant thyroid ablation with low dose radioiodine in Korean patients with low to intermediate-risk thyroid cancer. J Korean Med Sci. 30(7):876–81. DOI: 10.3346/jkms.2015.30.7.876. PMID: 26130949. PMCID: PMC4479940.
Article44. Sohn SY, Choi JY, Jang HW, Kim HJ, Jin SM, Kim SW, et al. 2013; Association between excessive urinary iodine excretion and failure of radioactive iodine thyroid ablation in patients with papillary thyroid cancer. Thyroid. 23(6):741–7. DOI: 10.1089/thy.2012.0136. PMID: 23205883. PMCID: PMC3675834.
Article45. Jeong JH, Kong EJ, Jeong SY, Lee SW, Cho IH, Ah Chun K, et al. 2017; Clinical outcomes of low-dose and high-dose postoperative radioiodine therapy in patients with intermediate- risk differentiated thyroid cancer. Nucl Med Commun. 38(3):228–33. DOI: 10.1097/MNM.0000000000000636. PMID: 27984538.46. Kwon SY, Lee SW, Kong EJ, Kim K, Kim BI, Kim J, et al. 2020; Clinicopathologic risk factors of radioactive iodine therapy based on response assessment in patients with differentiated thyroid cancer: a multicenter retrospective cohort study. Eur J Nucl Med Mol Imaging. 47(3):561–71. DOI: 10.1007/s00259-019-04634-8. PMID: 31820047.
Article47. Blumhardt R, Wolin EA, Phillips WT, Salman UA, Walker RC, Stack BC Jr, et al. 2014; Current controversies in the initial post-surgical radioactive iodine therapy for thyroid cancer: a narrative review. Endocr Relat Cancer. 21(6):R473–84. DOI: 10.1530/ERC-14-0286. PMID: 25277792.
Article48. Haddad RI, Bischoff L, Salgado SA, Applewhite M, Bernet V, Blomain E, et al. NCCN clinical practice guidelines in oncology (NCCN Guidelines®): Thyroid carcinoma. version 1.2024. Available from: URL: https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf.49. Jeong SY, Lee SW, Kim WW, Jung JH, Lee WK, Ahn BC, et al. 2019; Clinical outcomes of patients with T4 or N1b well-differentiated thyroid cancer after different strategies of adjuvant radioiodine therapy. Sci Rep. 9(1):5570. DOI: 10.1038/s41598-019-42083-3. PMID: 30944403. PMCID: PMC6447529.
Article50. Deandreis D, Rubino C, Tala H, Leboulleux S, Terroir M, Baudin E, et al. 2017; Comparison of empiric versus whole-body/-blood clearance dosimetry-based approach to radioactive iodine treatment in patients with metastases from differentiated thyroid cancer. J Nucl Med. 58(5):717–22. DOI: 10.2967/jnumed.116.179606. PMID: 27738010.
Article51. Park S, Bang JI, Kim K, Seo Y, Chong A, Hong CM, et al. 2024; Comparison of recombinant human thyroid-stimulating hormone and thyroid hormone withdrawal for 131 I therapy in patients with intermediate- to high-risk thyroid cancer : a systematic review and meta-analysis. Clin Nucl Med. 49(3):e96–e104. DOI: 10.1097/RLU.0000000000005022. PMID: 38271262.52. Borget I, Bonastre J, Catargi B, Deandreis D, Zerdoud S, Rusu D, et al. 2015; Quality of life and cost-effectiveness assessment of radioiodine ablation strategies in patients with thyroid cancer: results from the randomized phase III ESTIMABL trial. J Clin Oncol. 33(26):2885–92. DOI: 10.1200/JCO.2015.61.6722. PMID: 26240230.
Article53. Lee J, Yun MJ, Nam KH, Chung WY, Soh EY, Park CS. 2010; Quality of life and effectiveness comparisons of thyroxine withdrawal, triiodothyronine withdrawal, and recombinant thyroid-stimulating hormone administration for low-dose radioiodine remnant ablation of differentiated thyroid carcinoma. Thyroid. 20(2):173–9. DOI: 10.1089/thy.2009.0187. PMID: 20151824.
Article54. Coerts HI, de Keizer B, Marlowe RJ, Verburg FA. 2023; Recombinant or endogenous thyroid-stimulating hormone for radioactive iodine therapy in thyroid cancer: state of knowledge and current controversies. Eur J Endocrinol. 188(2):lvad006. DOI: 10.1093/ejendo/lvad006. PMID: 36655579.
Article55. Robbins RJ, Driedger A, Magner J. U.S. and Canadian Thyrogen Compassionate Use Program Investigator Group. 2006; Recombinant human thyrotropin-assisted radioiodine therapy for patients with metastatic thyroid cancer who could not elevate endogenous thyrotropin or be withdrawn from thyroxine. Thyroid. 16(11):1121–30. DOI: 10.1089/thy.2006.16.1121. PMID: 17123339.
Article56. Hershman JM, Edwards CL. 1972; Serum thyrotropin (TSH) levels after thyroid ablation compared with TSH levels after exogenous bovine TSH: implications for 131-I treatment of thyroid carcinoma. J Clin Endocrinol Metab. 34(5):814–8. DOI: 10.1210/jcem-34-5-814. PMID: 5062451.57. Hilts SV, Hellman D, Anderson J, Woolfenden J, Van Antwerp J, Patton D. 1979; Serial TSH determination after T3 withdrawal or thyroidectomy in the therapy of thyroid carcinoma. J Nucl Med. 20(9):928–32.58. Martin ND. 1978; Endogenous serum TSH levels and metastatic survey scans in thyroid cancer patients using triiodothyronine withdrawal. Clin Nucl Med. 3(10):401–3. DOI: 10.1097/00003072-197810000-00009. PMID: 729314.
Article59. Goldman JM, Line BR, Aamodt RL, Robbins J. 1980; Influence of triiodothyronine withdrawal time on 131I uptake postthyroidectomy for thyroid cancer. J Clin Endocrinol Metab. 50(4):734–9. DOI: 10.1210/jcem-50-4-734. PMID: 7364930.60. Schneider AB, Line BR, Goldman JM, Robbins J. 1981; Sequential serum thyroglobulin determinations, 131I scans, and 131I uptakes after triiodothyronine withdrawal in patients with thyroid cancer. J Clin Endocrinol Metab. 53(6):1199–206. DOI: 10.1210/jcem-53-6-1199. PMID: 7298799.61. Maxon HR, Thomas SR, Hertzberg VS, Kereiakes JG, Chen IW, Sperling MI, et al. 1983; Relation between effective radiation dose and outcome of radioiodine therapy for thyroid cancer. N Engl J Med. 309(16):937–41. DOI: 10.1056/NEJM198310203091601. PMID: 6621620.
Article62. Liel Y. 2002; Preparation for radioactive iodine administration in differentiated thyroid cancer patients. Clin Endocrinol (Oxf). 57(4):523–7. DOI: 10.1046/j.1365-2265.2002.01631.x. PMID: 12354135.
Article63. Sanchez R, Espinosa-de-los-Monteros AL, Mendoza V, Brea E, Hernandez I, Sosa E, et al. 2002; Adequate thyroid-stimulating hormone levels after levothyroxine discontinuation in the follow-up of patients with well-differentiated thyroid carcinoma. Arch Med Res. 33(5):478–81. DOI: 10.1016/S0188-4409(02)00394-6. PMID: 12459319.
Article64. Grigsby PW, Siegel BA, Bekker S, Clutter WE, Moley JF. 2004; Preparation of patients with thyroid cancer for 131I scintigraphy or therapy by 1-3 weeks of thyroxine discontinuation. J Nucl Med. 45(4):567–70.65. Serhal DI, Nasrallah MP, Arafah BM. 2004; Rapid rise in serum thyrotropin concentrations after thyroidectomy or withdrawal of suppressive thyroxine therapy in preparation for radioactive iodine administration to patients with differentiated thyroid cancer. J Clin Endocrinol Metab. 89(7):3285–9. DOI: 10.1210/jc.2003-031139. PMID: 15240604.
Article66. Leboeuf R, Perron P, Carpentier AC, Verreault J, Langlois MF. 2007; L-T3 preparation for whole-body scintigraphy: a randomized- controlled trial. Clin Endocrinol (Oxf). 67(6):839–44. DOI: 10.1111/j.1365-2265.2007.02972.x. PMID: 17645577.67. Edmonds CJ, Hayes S, Kermode JC, Thompson BD. 1977; Measurement of serum TSH and thyroid hormones in the management of treatment of thyroid carcinoma with radioiodine. Br J Radiol. 50(599):799–807. DOI: 10.1259/0007-1285-50-599-799. PMID: 588901.
Article68. Fallahi B, Beiki D, Takavar A, Fard-Esfahani A, Gilani KA, Saghari M, et al. 2012; Low versus high radioiodine dose in postoperative ablation of residual thyroid tissue in patients with differentiated thyroid carcinoma: a large randomized clinical trial. Nucl Med Commun. 33(3):275–82. DOI: 10.1097/MNM.0b013e32834e306a. PMID: 22124360.69. Prpic M, Dabelic N, Stanicic J, Jukic T, Milosevic M, Kusic Z. 2012; Adjuvant thyroid remnant ablation in patients with differentiated thyroid carcinoma confined to the thyroid: a comparison of ablation success with different activities of radioiodine (I-131). Ann Nucl Med. 26(9):744–51. DOI: 10.1007/s12149-012-0637-9. PMID: 22829399.
Article70. Karam M, Gianoukakis A, Feustel PJ, Cheema A, Postal ES, Cooper JA. 2003; Influence of diagnostic and therapeutic doses on thyroid remnant ablation rates. Nucl Med Commun. 24(5):489–95. DOI: 10.1097/00006231-200305000-00002. PMID: 12717064.
Article71. Vrachimis A, Riemann B, Mader U, Reiners C, Verburg FA. 2016; Endogenous TSH levels at the time of (131)I ablation do not influence ablation success, recurrence-free survival or differentiated thyroid cancer-related mortality. Eur J Nucl Med Mol Imaging. 43(2):224–31. DOI: 10.1007/s00259-015-3223-2. PMID: 26493309.
Article72. Ju N, Hou L, Song H, Qiu Z, Wang Y, Sun Z, et al. 2023; TSH >/=30 mU/L may not be necessary for successful 131I remnant ablation in patients with differentiated thyroid cancer. Eur Thyroid J. 12(4):e220219. DOI: 10.1530/ETJ-22-0219. PMID: 37022724. PMCID: PMC10305696.
Article73. Pluijmen MJ, Eustatia-Rutten C, Goslings BM, Stokkel MP, Arias AM, Diamant M, et al. 2003; Effects of low-iodide diet on postsurgical radioiodide ablation therapy in patients with differentiated thyroid carcinoma. Clin Endocrinol (Oxf). 58(4):428–35. DOI: 10.1046/j.1365-2265.2003.01735.x. PMID: 12641625.
Article74. Maxon HR, Thomas SR, Boehringer A, Drilling J, Sperling MI, Sparks JC, et al. 1983; Low iodine diet in I-131 ablation of thyroid remnants. Clin Nucl Med. 8(3):123–6. DOI: 10.1097/00003072-198303000-00006. PMID: 6851357.
Article75. Goslings BM. 1975; Proceedings: Effect of a low iodine diet on 131-I therapy in follicular thyroid carcinomata. J Endocrinol. 64(3):30P.76. Li JH, He ZH, Bansal V, Hennessey JV. 2016; Low iodine diet in differentiated thyroid cancer: a review. Clin Endocrinol (Oxf). 84(1):3–12. DOI: 10.1111/cen.12846. PMID: 26118628.
Article77. Lee M, Lee YK, Jeon TJ, Chang HS, Kim BW, Lee YS, et al. 2014; Low iodine diet for one week is sufficient for adequate preparation of high dose radioactive iodine ablation therapy of differentiated thyroid cancer patients in iodine-rich areas. Thyroid. 24(8):1289–96. DOI: 10.1089/thy.2013.0695. PMID: 24731156.
Article78. Herbert G, England C, Perry R, Whitmarsh A, Moore T, Searle A, et al. 2022; Impact of low iodine diets on ablation success in differentiated thyroid cancer: a mixed-methods systematic review and meta-analysis. Clin Endocrinol (Oxf). 97(6):702–29. DOI: 10.1111/cen.14751. PMID: 35484696. PMCID: PMC9790217.
Article79. Yoo ID, Kim SH, Seo YY, Oh JK, O JH, Chung SK. 2012; The success rate of initial (131)I ablation in differentiated thyroid cancer: comparison between less strict and very strict low iodine diets. Nucl Med Mol Imaging. 46(1):34–40. DOI: 10.1007/s13139-011-0111-y. PMID: 24900030. PMCID: PMC4042982.
Article80. Ju DL, Park YJ, Paik HY, Song Y. 2015; The impact of low adherence to the low-iodine diet on the efficacy of the radioactive iodine ablation therapy. Clin Nutr Res. 4(4):267–71. DOI: 10.7762/cnr.2015.4.4.267. PMID: 26566522. PMCID: PMC4641989.
Article81. Park JT 2nd, Hennessey JV. 2004; Two-week low iodine diet is necessary for adequate outpatient preparation for radioiodine rhTSH scanning in patients taking levothyroxine. Thyroid. 14(1):57–63. DOI: 10.1089/105072504322783858. PMID: 15009915.
Article82. Tomoda C, Uruno T, Takamura Y, Ito Y, Miya A, Kobayashi K, et al. 2005; Reevaluation of stringent low iodine diet in outpatient preparation for radioiodine examination and therapy. Endocr J. 52(2):237–40. DOI: 10.1507/endocrj.52.237. PMID: 15863954.
Article83. Morris LF, Wilder MS, Waxman AD, Braunstein GD. 2001; Reevaluation of the impact of a stringent low-iodine diet on ablation rates in radioiodine treatment of thyroid carcinoma. Thyroid. 11(8):749–55. DOI: 10.1089/10507250152484583. PMID: 11525267.
Article84. Kim HK, Lee SY, Lee JI, Jang HW, Kim SK, Chung HS, et al. 2011; Daily urine iodine excretion while consuming a low-iodine diet in preparation for radioactive iodine therapy in a high iodine intake area. Clin Endocrinol (Oxf). 75(6):851–6. DOI: 10.1111/j.1365-2265.2011.04157.x. PMID: 21707689.
Article85. Fatourechi V, Hay ID, Mullan BP, Wiseman GA, Eghbali- Fatourechi GZ, Thorson LM, et al. 2000; Are posttherapy radioiodine scans informative and do they influence subsequent therapy of patients with differentiated thyroid cancer? Thyroid. 10(7):573–7. DOI: 10.1089/thy.2000.10.573. PMID: 10958309.
Article86. Sherman SI, Tielens ET, Sostre S, Wharam MD Jr, Ladenson PW. 1994; Clinical utility of posttreatment radioiodine scans in the management of patients with thyroid carcinoma. J Clin Endocrinol Metab. 78(3):629–34. DOI: 10.1210/jcem.78.3.8126134. PMID: 8126134.
Article87. Souza Rosario PW, Barroso AL, Rezende LL, Padrao EL, Fagundes TA, Penna GC, et al. 2004; Post I-131 therapy scanning in patients with thyroid carcinoma metastases: an unnecessary cost or a relevant contribution? Clin Nucl Med. 29(12):795–8. DOI: 10.1097/00003072-200412000-00005. PMID: 15545881.
Article88. Ciappuccini R, Heutte N, Trzepla G, Rame JP, Vaur D, Aide N, et al. 2011; Postablation (131)I scintigraphy with neck and thorax SPECT-CT and stimulated serum thyroglobulin level predict the outcome of patients with differentiated thyroid cancer. Eur J Endocrinol. 164(6):961–9. DOI: 10.1530/EJE-11-0156. PMID: 21471170.
Article89. Kohlfuerst S, Igerc I, Lobnig M, Gallowitsch HJ, Gomez- Segovia I, Matschnig S, et al. 2009; Posttherapeutic (131)I SPECT- CT offers high diagnostic accuracy when the findings on conventional planar imaging are inconclusive and allows a tailored patient treatment regimen. Eur J Nucl Med Mol Imaging. 36(6):886–93. DOI: 10.1007/s00259-008-1044-2. PMID: 19169681.
Article90. Chen L, Luo Q, Shen Y, Yu Y, Yuan Z, Lu H, et al. 2008; Incremental value of 131I SPECT/CT in the management of patients with differentiated thyroid carcinoma. J Nucl Med. 49(12):1952–7. DOI: 10.2967/jnumed.108.052399. PMID: 18997044.
Article91. Schmidt D, Linke R, Uder M, Kuwert T. 2010; Five months' follow-up of patients with and without iodine-positive lymph node metastases of thyroid carcinoma as disclosed by (131)I- SPECT/CT at the first radioablation. Eur J Nucl Med Mol Imaging. 37(4):699–705. DOI: 10.1007/s00259-009-1299-2. PMID: 19936746.
Article92. Maruoka Y, Abe K, Baba S, Isoda T, Sawamoto H, Tanabe Y, et al. 2012; Incremental diagnostic value of SPECT/CT with 131I scintigraphy after radioiodine therapy in patients with well- differentiated thyroid carcinoma. Radiology. 265(3):902–9. DOI: 10.1148/radiol.12112108. PMID: 23012466.
Article93. Grewal RK, Tuttle RM, Fox J, Borkar S, Chou JF, Gonen M, et al. 2010; The effect of posttherapy 131I SPECT/CT on risk classification and management of patients with differentiated thyroid cancer. J Nucl Med. 51(9):1361–7. DOI: 10.2967/jnumed.110.075960. PMID: 20720058.
Article94. Jeong SY, Lee SW, Kim HW, Song BI, Ahn BC, Lee J. 2014; Clinical applications of SPECT/CT after first I-131 ablation in patients with differentiated thyroid cancer. Clin Endocrinol (Oxf). 81(3):445–51. DOI: 10.1111/cen.12460. PMID: 24716874.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Patient Preparation and Special Consideration before Radioactive Iodine Therapy
- Differentiated Thyroid Cancer and Radioactive Iodine: Past, Present and Future
- New Trends in Radioiodine Treatment for the Advanced Differentiated Thyroid Cancer
- Diagnostic and Therapeutic Approaches to Radioactive Iodine Refractory Differentiated Thyroid Cancer
- Postoperative Follow-Up of Differentiated Thyroid Cancer: Use of Thyroglobulin Assay