J Korean Med Sci.
2024 Jun;39(21):e174. 10.3346/jkms.2024.39.e174.
Vaccine Effectiveness Against Severe Acute Respiratory Syndrome Coronavirus 2 Reinfection by Type and Frequency of Vaccine: A CommunityBased Case-Control Study
- Affiliations
-
- 1Infectious Disease Research Center, Citizens’ Health Bureau, Seoul Metropolitan Government, Seoul, Korea
- 2Department of Epidemiology & Health Informatics, Graduate School of Public Health, Korea University, Seoul, Korea
- 3Department of Preventive Medicine, Korea University College of Medicine, Seoul, Korea
- KMID: 2556290
- DOI: http://doi.org/10.3346/jkms.2024.39.e174
Abstract
- Background
Although guidelines recommend vaccination for individuals who have recovered from the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection to prevent reinfection, comprehensive evaluation studies are limited. We aimed to evaluate vaccine effectiveness against SARS-CoV-2 reinfection according to the primary vaccination status, booster vaccination status, and vaccination methods used.
Methods
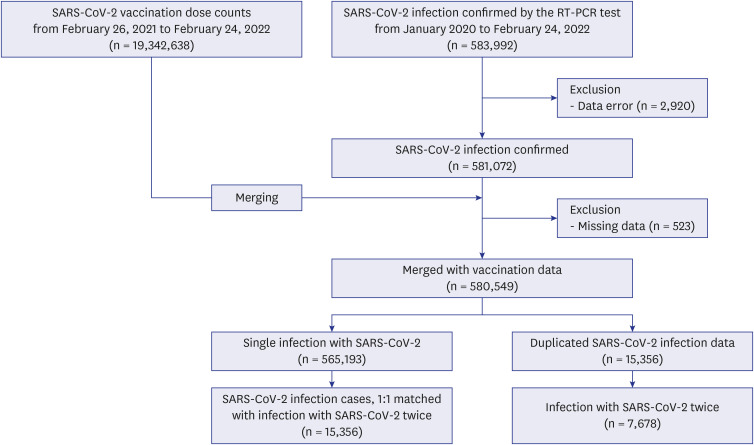
This population-based case-control study enrolled all SARS-CoV-2-infected patients in Seoul between January 2020 and February 2022. Individuals were categorized into case (reinfection) and control (no reinfection) groups. Data were analyzed using conditional logistic regression after adjusting for underlying comorbidities using multiple regression.
Results
The case group included 7,678 participants (average age: 32.26 years). In all vaccinated individuals, patients who received the first and second booster doses showed reduced reinfection rates compared with individuals who received basic vaccination (odds ratio [OR] = 0.605, P < 0.001 and OR = 0.002, P < 0.001). Patients who received BNT162b2 or mRNA-1273, NVX-CoV2373 and heterologous vaccination showed reduced reinfection rates compared with unvaccinated individuals (OR = 0.546, P < 0.001; OR = 0.356, P < 0.001; and OR = 0.472, P < 0.001). However, the ChAdOx1-S or Ad26.COV2.S vaccination group showed a higher reinfection rate than the BNT162b2 or mRNA-1273 vaccination group (OR = 4.419, P < 0.001).
Conclusion
In SARS-CoV-2-infected individuals, completion of the basic vaccination series showed significant protection against reinfection compared with no vaccination. If the first or second booster vaccination was received, the protective effect against reinfection was higher than that of basic vaccination; when vaccinated with BNT162b2 or mRNA-1273 only or heterologous vaccination, the protective effect was higher than that of ChAdOx1-S or Ad26. COV2.S vaccination only.
Keyword
Figure
Reference
-
1. Gram MA, Emborg HD, Schelde AB, Friis NU, Nielsen KF, Moustsen-Helms IR, et al. Vaccine effectiveness against SARS-CoV-2 infection or COVID-19 hospitalization with the alpha, delta, or omicron SARS-CoV-2 variant: a nationwide Danish cohort study. PLoS Med. 2022; 19(9):e1003992. PMID: 36048766.2. Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med. 2021; 385(7):585–594. PMID: 34289274.3. Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022; 386(16):1532–1546. PMID: 35249272.4. Link-Gelles R, Ciesla AA, Fleming-Dutra KE, Smith ZR, Britton A, Wiegand RE, et al. Effectiveness of bivalent mRNA vaccines in preventing symptomatic SARS-CoV-2 infection—increasing community access to testing program, United States, September–November 2022. MMWR Morb Mortal Wkly Rep. 2022; 71(48):1526–1530. PMID: 36454688.5. Intawong K, Chariyalertsak S, Chalom K, Wonghirundecha T, Kowatcharakul W, Thongprachum A, et al. Effectiveness of heterologous third and fourth dose COVID-19 vaccine schedules for SARS-CoV-2 infection during delta and omicron predominance in Thailand: a test-negative, case-control study. Lancet Reg Health Southeast Asia. 2023; 10:100121. PMID: 36465090.6. Carazo S, Skowronski DM, Brisson M, Barkati S, Sauvageau C, Brousseau N, et al. Protection against omicron (B.1.1.529) BA.2 reinfection conferred by primary omicron BA.1 or pre-omicron SARS-CoV-2 infection among health-care workers with and without mRNA vaccination: a test-negative case-control study. Lancet Infect Dis. 2023; 23(1):45–55. PMID: 36152671.7. World Heath Organization (WHO). COVID-19 advice for the public: getting vaccinated. Accessed October 4, 2022. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice/ .8. Centers for Disease Control and Prevention (CDC). Stay up to date with COVID-19 vaccines. Accessed February 1, 2023. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/stay-up-to-date.html/ .9. Townsend JP, Hassler HB, Wang Z, Miura S, Singh J, Kumar S, et al. The durability of immunity against reinfection by SARS-CoV-2: a comparative evolutionary study. Lancet Microbe. 2021; 2(12):e666–e675. PMID: 34632431.10. Government Seoul Metropolitan Government. The daily news review about Seoul response to COVID-19, 2022. Accessed October 4, 2022. https://www.seoul.go.kr/coronaV/coronaStatus_old.do?menu_code=07/ .11. Arteaga-Livias K, Panduro-Correa V, Pinzas-Acosta K, Perez-Abad L, Pecho-Silva S, Espinoza-Sánchez F, et al. COVID-19 reinfection? A suspected case in a Peruvian patient. Travel Med Infect Dis. 2021; 39:101947. PMID: 33307196.12. Yahav D, Yelin D, Eckerle I, Eberhardt CS, Wang J, Cao B, et al. Definitions for coronavirus disease 2019 reinfection, relapse and PCR re-positivity. Clin Microbiol Infect. 2021; 27(3):315–318. PMID: 33285276.13. Abu-Raddad LJ, Chemaitelly H, Bertollini R. National Study Group for COVID-19 Epidemiology. Severity of SARS-CoV-2 reinfections as compared with primary infections. N Engl J Med. 2021; 385(26):2487–2489. PMID: 34818474.14. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021; 384(5):403–416. PMID: 33378609.15. Thomas SJ, Moreira ED Jr, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med. 2021; 385(19):1761–1773. PMID: 34525277.16. Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Safety and efficacy of single-dose Ad26. COV2. S vaccine against Covid-19. N Engl J Med. 2021; 384(23):2187–2201. PMID: 33882225.17. Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021; 397(10269):99–111. PMID: 33306989.18. Korea Disease Control and Prevention Agency. COVID-19 response guidelines. Accessed October 20, 2022. https://www.kdca.go.kr/filepath/boardSyview.es?bid=0019&list_no=718628&seq=2 .19. Bajgain KT, Badal S, Bajgain BB, Santana MJ. Prevalence of comorbidities among individuals with COVID-19: a rapid review of current literature. Am J Infect Control. 2021; 49(2):238–246. PMID: 32659414.20. Thakur B, Dubey P, Benitez J, Torres JP, Reddy S, Shokar N, et al. A systematic review and meta-analysis of geographic differences in comorbidities and associated severity and mortality among individuals with COVID-19. Sci Rep. 2021; 11(1):8562. PMID: 33879826.21. Honardoost M, Janani L, Aghili R, Emami Z, Khamseh ME. The association between presence of comorbidities and COVID-19 severity: a systematic review and meta-analysis. Cerebrovasc Dis. 2021; 50(2):132–140. PMID: 33530081.22. Korea Disease Control and Prevention Agency. Revision of COVID-19 vaccination standards and related FAQ information. Accessed September 27, 2022. https://kdca.go.kr/filepath/boardSyview.es?bid=0019&list_no=720112&seq=1 .23. Noor FM, Islam MM. Prevalence and associated risk factors of mortality among COVID-19 patients: a meta-analysis. J Community Health. 2020; 45(6):1270–1282. PMID: 32918645.24. Hammerman A, Sergienko R, Friger M, Beckenstein T, Peretz A, Netzer D, et al. Effectiveness of the BNT162b2 vaccine after recovery from Covid-19. N Engl J Med. 2022; 386(13):1221–1229. PMID: 35172072.25. Carazo S, Skowronski DM, Brisson M, Sauvageau C, Brousseau N, Gilca R, et al. Estimated protection of prior SARS-CoV-2 infection against reinfection with the omicron variant among messenger RNA–vaccinated and nonvaccinated individuals in Quebec, Canada. JAMA Netw Open. 2022; 5(10):e2236670. PMID: 36239934.26. Kim J, Choe YJ, Jang EJ, Lim DS, Kim YY, Kim RK, et al. Effectiveness of booster mRNA vaccines against SARS-CoV-2 infection in an elderly population, South Korea, October 2021–January 2022. Clin Infect Dis. 2022; 75(5):920–921. PMID: 35439294.27. Gazit S, Saciuk Y, Perez G, Peretz A, Pitzer VE, Patalon T. Short term, relative effectiveness of four doses versus three doses of BNT162b2 vaccine in people aged 60 years and older in Israel: retrospective, test negative, case-control study. BMJ. 2022; 377:e071113. PMID: 35609888.28. Chemaitelly H, Ayoub HH, AlMukdad S, Coyle P, Tang P, Yassine HM, et al. Duration of mRNA vaccine protection against SARS-CoV-2 omicron BA.1 and BA.2 subvariants in Qatar. Nat Commun. 2022; 13(1):3082. PMID: 35654888.29. Lin DY, Gu Y, Xu Y, Wheeler B, Young H, Sunny SK, et al. Association of primary and booster vaccination and prior infection with SARS-CoV-2 infection and severe COVID-19 outcomes. JAMA. 2022; 328(14):1415–1426. PMID: 36155617.30. Bánki Z, Mateus J, Rössler A, Schäfer H, Bante D, Riepler L, et al. Heterologous ChAdOx1/BNT162b2 vaccination induces stronger immune response than homologous ChAdOx1 vaccination: The pragmatic, multi-center, three-arm, partially randomized HEVACC trial. EBioMedicine. 2022; 80:104073. PMID: 35617826.31. Corrao G, Franchi M, Cereda D, Bortolan F, Zoli A, Leoni O, et al. Persistence of protection against SARS-CoV-2 clinical outcomes up to 9 months since vaccine completion: a retrospective observational analysis in Lombardy, Italy. Lancet Infect Dis. 2022; 22(5):649–656. PMID: 35093194.32. Accorsi EK, Britton A, Shang N, Fleming-Dutra KE, Link-Gelles R, Smith ZR, et al. Effectiveness of homologous and heterologous Covid-19 boosters against omicron. N Engl J Med. 2022; 386(25):2433–2435. PMID: 35613039.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current updates and research on plant-based vaccines for coronavirus disease 2019
- Thrombosis and severe acute respiratory syndrome coronavirus 2 vaccines: vaccine-induced immune thrombotic thrombocytopenia
- Review of COVID-19 Vaccines and Their Evidence in Older Adults
- Epidemiology, Virology, and Clinical Features of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2; Coronavirus Disease-19)
- Effectiveness of Heterologous COVID-19 Vaccine Booster in Korean Elderly Population, 2022