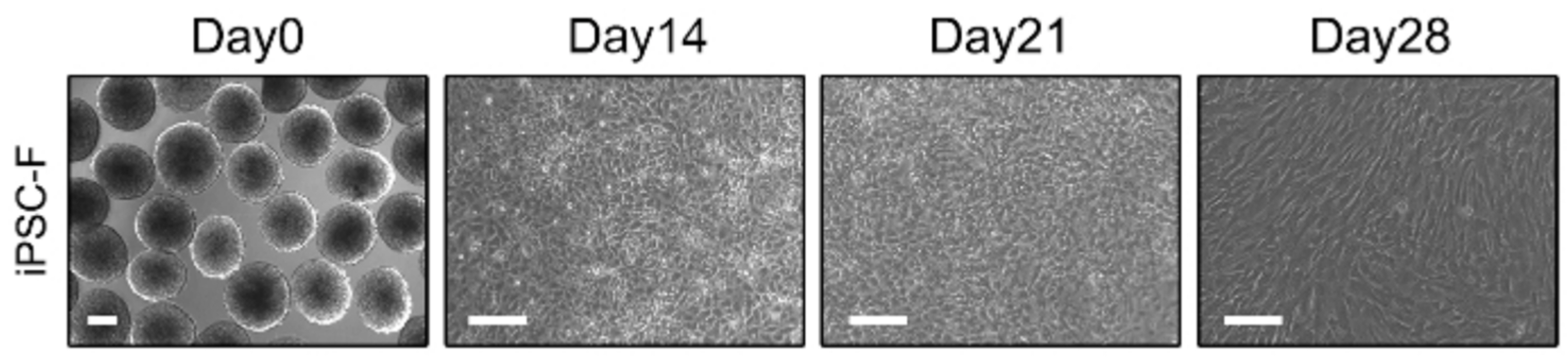
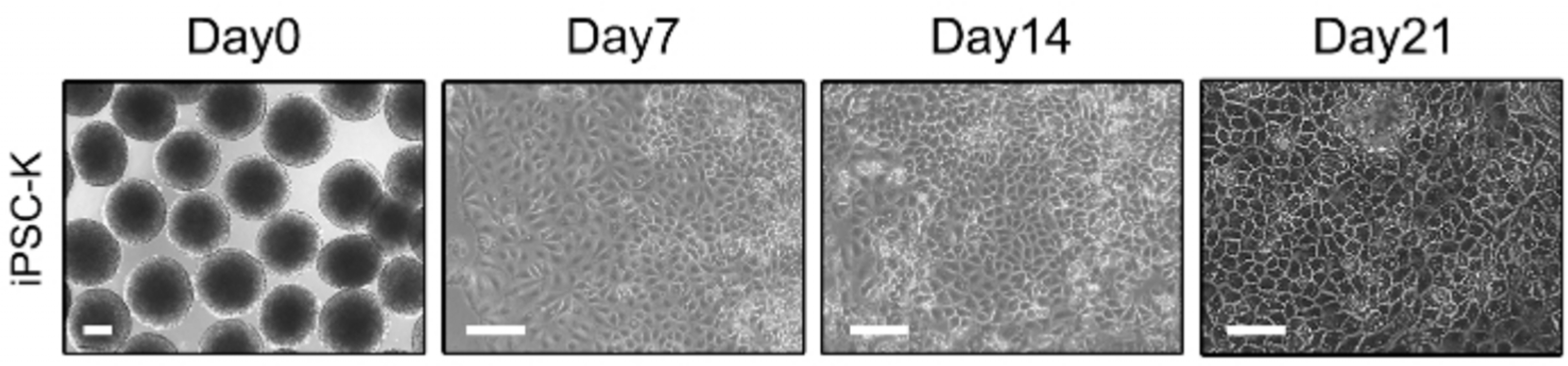
Int J Stem Cells.
2024 May;17(2):182-193. 10.15283/ijsc24045.
Guidelines for Manufacturing and Application of Organoids: Skin
- Affiliations
-
- 1Organoid Standards Initiative
- 2Kangstem Biotech Co., Ltd., Seoul, Korea
- 3Catholic Induced Pluripotent Stem Cell Research Center (CiSTEM), Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 4Department of Biomedicine & Health Sciences, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 5YiPSCELL Inc., Seoul, Korea
- 6Biosolution Co., Ltd., Seoul, Korea
- 7CellinCells, Seoul National University Dental Hospital, Seoul, Korea
- 8Department of Biophysics, Sungkyunkwan University, Suwon, Korea
- 9Institute of Quantum Biophysics, Sungkyunkwan University, Suwon, Korea
- 10Division of Rheumatology, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2556236
- DOI: http://doi.org/10.15283/ijsc24045
Abstract
- To address the limitations of animal testing, scientific research is increasingly focused on developing alternative testing methods. These alternative tests utilize cells or tissues derived from animals or humans for in vitro testing, as well as artificial tissues and organoids. In western countries, animal testing for cosmetics has been banned, leading to the adoption of artificial skin for toxicity evaluation, such as skin corrosion and irritation assessments. Standard guidelines for skin organoid technology becomes necessary to ensure consistent data and evaluation in replacing animal testing with in vitro methods. These guidelines encompass aspects such as cell sourcing, culture techniques, quality requirements and assessment, storage and preservation, and organoid-based assays.
Figure
Cited by 1 articles
-
Standards for Organoids
Sun-Ju Ahn
Int J Stem Cells. 2024;17(2):99-101. doi: 10.15283/ijsc24043.
Reference
-
References
1. Jung SY, You HJ, Kim MJ, Ko G, Lee S, Kang KS. 2022; Wnt-activating human skin organoid model of atopic dermatitis induced by Staphylococcus aureus and its protective effects by Cutibacterium acnes. iScience. 25:105150. DOI: 10.1016/j.isci.2022.105150. PMID: 36193049. PMCID: PMC9526179.2. Corsini NS, Knoblich JA. 2022; Human organoids: new strategies and methods for analyzing human development and disease. Cell. 185:2756–2769. DOI: 10.1016/j.cell.2022.06.051. PMID: 35868278.
Article3. Wang Y, Wang P, Qin J. 2022; Human organoids and organs-on-chips for addressing COVID-19 challenges. Adv Sci (Weinh). 9:e2105187. DOI: 10.1002/advs.202105187. PMID: 35107217. PMCID: PMC8981475.
Article4. Silva RJ, Tamburic S. 2022; A state-of-the-art review on the alternatives to animal testing for the safety assessment of cosmetics. Cosmetics. 9:90. DOI: 10.3390/cosmetics9050090.
Article5. Wang M, Zhang L, Hao H, Yan M, Zhu Z. 2024; Applications of engineered skin tissue for cosmetic component and toxicology detection. Cell Transplant. 33:9636897241235464. DOI: 10.1177/09636897241235464. PMID: 38491929. PMCID: PMC10944590.
Article6. Deshmukh GR, Kumar KH, Reddy PV, Rao BS. 2012; In vitro skin corrosion: human skin model test - a validation study. Toxicol In Vitro. 26:1072–1074. DOI: 10.1016/j.tiv.2012.04.021. PMID: 22542585.7. OECD. 2023. Test No. 498: in vitro phototoxicity - reconstructed human epidermis phototoxicity test method. OECD.8. OECD. 2021. Test No. 439: in vitro skin irritation: reconstructed human epidermis test method. OECD.9. OECD. 2019. Test No. 431: in vitro skin corrosion: reconstructed human epidermis (RHE) test method. OECD.10. Moniz T, Costa Lima SA, Reis S. 2020; Human skin models: from healthy to disease-mimetic systems; characteristics and applications. Br J Pharmacol. 177:4314–4329. DOI: 10.1111/bph.15184. PMID: 32608012. PMCID: PMC7484561.
Article11. Lee J, Koehler KR. 2021; Skin organoids: a new human model for developmental and translational research. Exp Dermatol. 30:613–620. DOI: 10.1111/exd.14292. PMID: 33507537. PMCID: PMC8265774.
Article12. Lee J, Rabbani CC, Gao H, et al. 2020; Hair-bearing human skin generated entirely from pluripotent stem cells. Nature. 582:399–404. DOI: 10.1038/s41586-020-2352-3. PMID: 32494013. PMCID: PMC7593871.
Article13. Kim J, Koo BK, Knoblich JA. 2020; Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol. 21:571–584. DOI: 10.1038/s41580-020-0259-3. PMID: 32636524. PMCID: PMC7339799.
Article14. Clevers H. 2016; Modeling development and disease with organoids. Cell. 165:1586–1597. DOI: 10.1016/j.cell.2016.05.082. PMID: 27315476.
Article15. Fujii M, Sato T. 2021; Somatic cell-derived organoids as prototypes of human epithelial tissues and diseases. Nat Mater. 20:156–169. DOI: 10.1038/s41563-020-0754-0. PMID: 32807924.
Article16. Sato T, Vries RG, Snippert HJ, et al. 2009; Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 459:262–265. DOI: 10.1038/nature07935. PMID: 19329995.
Article17. Wang Y, Lin H, Zhao L, et al. 2023; Standard: human intestinal organoids. Cell Regen. 12:23. DOI: 10.1186/s13619-023-00168-5. PMID: 37314549. PMCID: PMC10267020.18. Sun H, Zhang YX, Li YM. 2021; Generation of skin organoids: potential opportunities and challenges. Front Cell Dev Biol. 9:709824. DOI: 10.3389/fcell.2021.709824. PMID: 34805138. PMCID: PMC8600117.
Article19. Ganesh K, Wu C, O'Rourke KP, et al. 2019; A rectal cancer organoid platform to study individual responses to chemoradiation. Nat Med. 25:1607–1614. DOI: 10.1038/s41591-019-0584-2. PMID: 31591597. PMCID: PMC7385919.
Article20. de Jongh D, Massey EK, Bunnik EM. VANGUARD consortium. 2022; Organoids: a systematic review of ethical issues. Stem Cell Res Ther. 13:337. DOI: 10.1186/s13287-022-02950-9. PMID: 35870991. PMCID: PMC9308907.
Article21. Geraghty RJ, Capes-Davis A, Davis JM, et al. 2014; Guidelines for the use of cell lines in biomedical research. Br J Cancer. 111:1021–1046. DOI: 10.1038/bjc.2014.166. PMID: 25117809. PMCID: PMC4453835.
Article22. Sullivan S, Stacey GN, Akazawa C, et al. 2018; Quality control guidelines for clinical-grade human induced pluripotent stem cell lines. Regen Med. 13:859–866. DOI: 10.2217/rme-2018-0095. PMID: 30205750.
Article23. Steeg R, Mueller SC, Mah N, et al. 2021; EBiSC best practice: how to ensure optimal generation, qualification, and distribution of iPSC lines. Stem Cell Reports. 16:1853–1867. DOI: 10.1016/j.stemcr.2021.07.009. PMID: 34380020. PMCID: PMC8365092.
Article24. Rohani L, Johnson AA, Naghsh P, Rancourt DE, Ulrich H, Holland H. 2018; Concise review: molecular cytogenetics and quality control: clinical guardians for pluripotent stem cells. Stem Cells Transl Med. 7:867–875. DOI: 10.1002/sctm.18-0087. PMID: 30218497. PMCID: PMC6265634.
Article25. Yin X, Mead BE, Safaee H, Langer R, Karp JM, Levy O. 2016; Engineering stem cell organoids. Cell Stem Cell. 18:25–38. DOI: 10.1016/j.stem.2015.12.005. PMID: 26748754. PMCID: PMC4728053.
Article26. Shang Z, Feng H, Xia L. 2023; The suppression effects of fat mass and obesity associated gene on the hair follicle-derived neural crest stem cells differentiating into melanocyte by N6-methyladenosine modifying microphthalmia-associated transcription factor. Int J Stem Cells. 16:135–144. DOI: 10.15283/ijsc22106. PMID: 36823977. PMCID: PMC10226864.
Article27. Zhao Z, Chen X, Dowbaj AM, et al. 2022; Organoids. Nat Rev Methods Primers. 2:94. DOI: 10.1038/s43586-022-00174-y. PMID: 37325195. PMCID: PMC10270325.
Article28. Kim Y, Ju JH. 2019; Generation of 3D skin organoid from cord blood-derived induced pluripotent stem cells. J Vis Exp. (146):e59297. DOI: 10.3791/59297.
Article29. Posch W, Lass-Flörl C, Wilflingseder D. 2016; Generation of human monocyte-derived dendritic cells from whole blood. J Vis Exp. (118):54968. DOI: 10.3791/54968. PMID: 28060313. PMCID: PMC5226452.
Article30. Sachamitr P, Leishman AJ, Davies TJ, Fairchild PJ. 2018; Directed differentiation of human induced pluripotent stem cells into dendritic cells displaying tolerogenic properties and resembling the CD141+ subset. Front Immunol. 8:1935. DOI: 10.3389/fimmu.2017.01935. PMID: 29358940. PMCID: PMC5766641.31. Yousef H, Alhajj M, Sharma S. 2024. Anatomy, skin (integument), epidermis. StatPearls Publishing;StatPearls:32. Caloni F, De Angelis I, Hartung T. 2022; Replacement of animal testing by integrated approaches to testing and assessment (IATA): a call for in vivitrosi. Arch Toxicol. 96:1935–1950. DOI: 10.1007/s00204-022-03299-x. PMID: 35503372. PMCID: PMC9151502.
Article33. Gądarowska D, Kalka J, Daniel-Wójcik A, Mrzyk I. 2022; Alternative methods for skin-sensitization assessment. Toxics. 10:740. DOI: 10.3390/toxics10120740.
Article34. Everett JS, Sommers MS. 2013; Skin viscoelasticity: physiologic mechanisms, measurement issues, and application to nursing science. Biol Res Nurs. 15:338–346. DOI: 10.1177/1099800411434151. PMID: 22544517. PMCID: PMC3465619.35. Furbo S, Urbano PCM, Raskov HH, Troelsen JT, Kanstrup Fiehn AM, Gögenur I. 2022; Use of patient-derived organoids as a treatment selection model for colorectal cancer: a narrative review. Cancers (Basel). 14:1069. DOI: 10.3390/cancers14041069. PMID: 35205817. PMCID: PMC8870458.
Article36. Han J, Kim S, Lee SH, et al. 2020; Me-too validation study for in vitro skin irritation test with a reconstructed human epidermis model, KeraSkinTM for OECD test guideline 439. Regul Toxicol Pharmacol. 117:104725. DOI: 10.1016/j.yrtph.2020.104725. PMID: 32768665.37. OECD. 2010. Test No. 429: skin sensitisation: local lymph node assay. OECD.38. OECD. 2010. Test No. 442A: skin sensitization: local lymph node assay: DA. OECD.39. OECD. 2018. Test No. 442B: skin sensitization: local lymph node assay: BrdU-ELISA or -FCM. OECD.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Essential Guidelines for Manufacturing and Application of Organoids
- Guidelines for Manufacturing and Application of Organoids: Heart
- Establishment and Advancement of Pancreatic Organoids
- Region Specific Brain Organoids to Study Neurodevelopmental Disorders
- Guidelines for Manufacturing and Application of Organoids: Liver