Diabetes Metab J.
2024 May;48(3):440-448. 10.4093/dmj.2023.0200.
Glycemic Control Is Associated with Histological Findings of Nonalcoholic Fatty Liver Disease
- Affiliations
-
- 1Department of Gastroenterology and Metabology, Ehime University Graduate School of Medicine, Toon, Japan
- 2Health Services Center, Ehime University, Matsuyama, Japan
- 3Department of Lifestyle-related Medicine and Endocrinology, Ehime University Graduate School of Medicine, Toon, Japan
- 4Postgraduate Medical Education Center, Ehime University Graduate School of Medicine, Toon, Japan
- KMID: 2555775
- DOI: http://doi.org/10.4093/dmj.2023.0200
Abstract
- Background
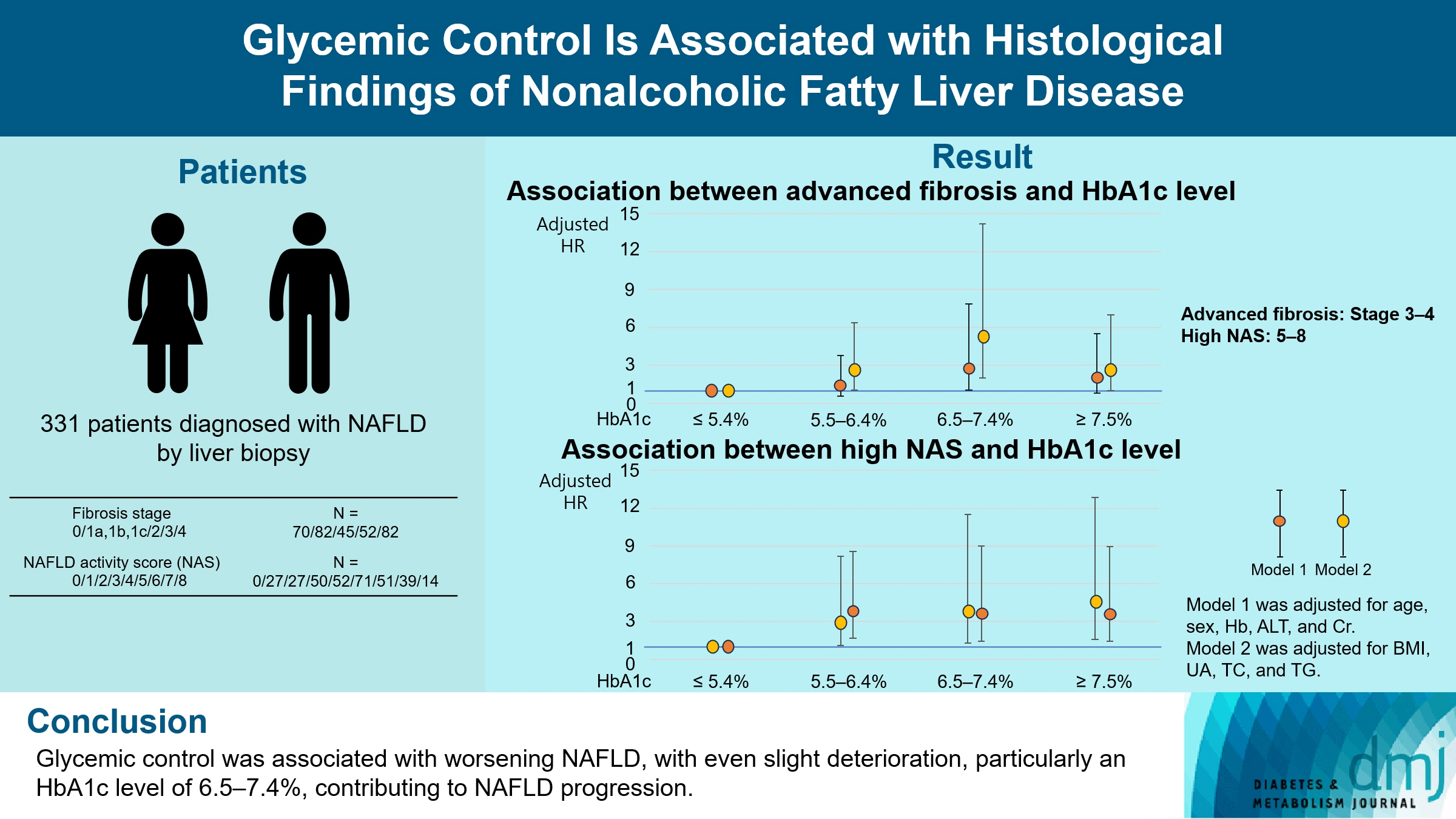
Poor lifestyle habits may worsen nonalcoholic fatty liver disease (NAFLD), with progression to nonalcoholic steatohepatitis (NASH) and cirrhosis. This study investigated the association between glycemic control status and hepatic histological findings to elucidate the effect of glycemic control on NAFLD.
Methods
This observational study included 331 patients diagnosed with NAFLD by liver biopsy. Effects of the glycemic control status on histological findings of NAFLD were evaluated by comparing the following four glycemic status groups defined by the glycosylated hemoglobin (HbA1c) level at the time of NAFLD diagnosis: ≤5.4%, 5.5%–6.4%, 6.5%–7.4%, and ≥7.5%.
Results
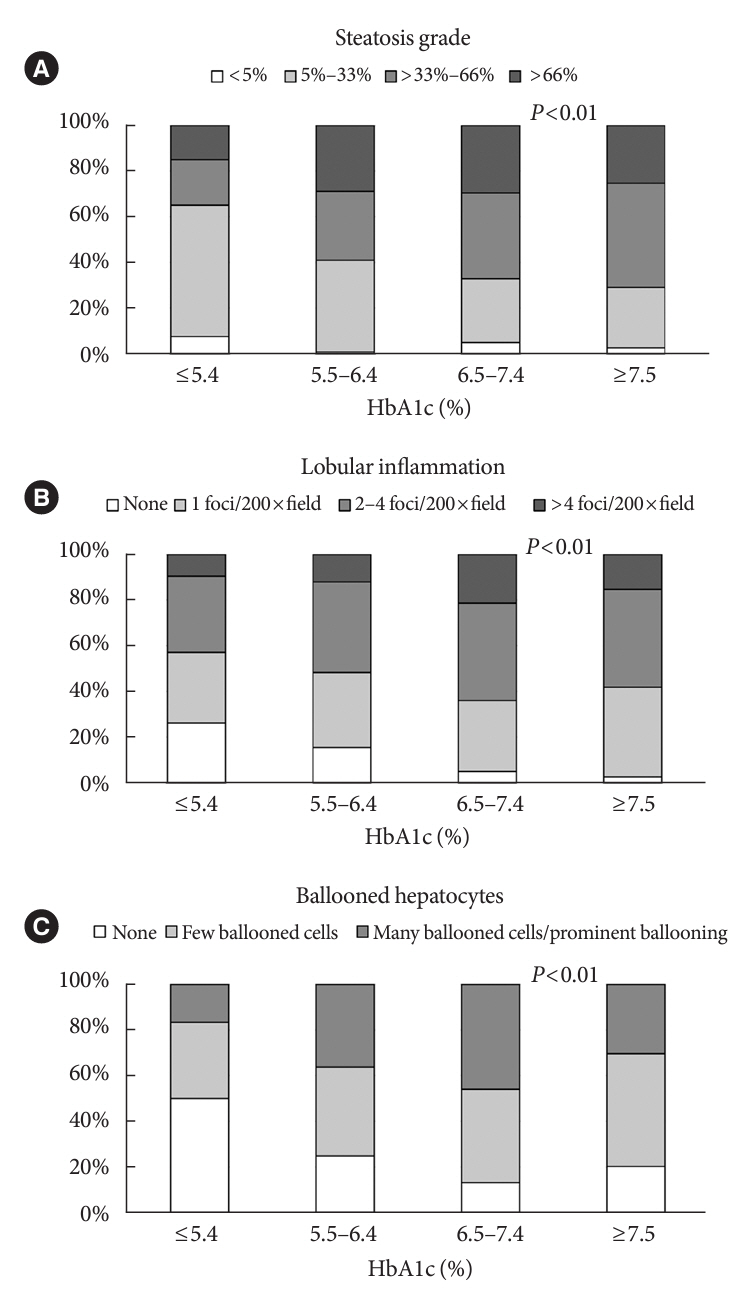
Compared with the lowest HbA1c group (≤5.4%), the higher HbA1c groups (5.5%–6.4%, 6.5%–7.4%, and ≥7.5%) were associated with advanced liver fibrosis and high NAFLD activity score (NAS). On multivariate analysis, an HbA1c level of 6.5%– 7.4% group was significantly associated with advanced fibrosis compared with the lowest HbA1c group after adjusting for age, sex, hemoglobin, alanine aminotransferase, and creatinine levels. When further controlling for body mass index and uric acid, total cholesterol, and triglyceride levels, the higher HbA1c groups were significantly associated with advanced fibrosis compared with the lowest HbA1c group. On the other hand, compared with the lowest HbA1c group, the higher HbA1c groups were also associated with a high NAS in both multivariate analyses.
Conclusion
Glycemic control is associated with NAFLD exacerbation, with even a mild deterioration in glycemic control, especially a HbA1c level of 6.5%–7.4%, contributing to NAFLD progression.
Figure
Reference
-
1. Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003; 37:917–23.
Article2. Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001; 50:1844–50.3. Sanyal AJ; American Gastroenterological Association. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002; 123:1705–25.
Article4. Tokushige K, Ikejima K, Ono M, Eguchi Y, Kamada Y, Itoh Y, et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis 2020. Hepatol Res. 2021; 51:1013–25.
Article5. Yoshioka Y, Hashimoto E, Yatsuji S, Kaneda H, Taniai M, Tokushige K, et al. Nonalcoholic steatohepatitis: cirrhosis, hepatocellular carcinoma, and burnt-out NASH. J Gastroenterol. 2004; 39:1215–8.
Article6. Shimada M, Hashimoto E, Taniai M, Hasegawa K, Okuda H, Hayashi N, et al. Hepatocellular carcinoma in patients with non-alcoholic steatohepatitis. J Hepatol. 2002; 37:154–60.
Article7. Mahfood Haddad T, Hamdeh S, Kanmanthareddy A, Alla VM. Nonalcoholic fatty liver disease and the risk of clinical cardiovascular events: a systematic review and meta-analysis. Diabetes Metab Syndr. 2017; 11 Suppl 1:S209–16.
Article8. Ekstedt M, Hagstrom H, Nasr P, Fredrikson M, Stal P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015; 61:1547–54.
Article9. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004; 27:1047–53.
Article10. Partanen J, Niskanen L, Lehtinen J, Mervaala E, Siitonen O, Uusitupa M. Natural history of peripheral neuropathy in patients with non-insulin-dependent diabetes mellitus. N Engl J Med. 1995; 333:89–94.
Article11. Leon BM, Maddox TM. Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes. 2015; 6:1246–58.
Article12. Shima T, Uto H, Ueki K, Kohgo Y, Yasui K, Nakamura N, et al. Hepatocellular carcinoma as a leading cause of cancer-related deaths in Japanese type 2 diabetes mellitus patients. J Gastroenterol. 2019; 54:64–77.
Article13. McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015; 62:1148–55.
Article14. Nakahara T, Hyogo H, Yoneda M, Sumida Y, Eguchi Y, Fujii H, et al. Type 2 diabetes mellitus is associated with the fibrosis severity in patients with nonalcoholic fatty liver disease in a large retrospective cohort of Japanese patients. J Gastroenterol. 2014; 49:1477–84.
Article15. Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015; 149:389–97.
Article16. Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol. 2017; 14:32–42.
Article17. Hamaguchi E, Takamura T, Sakurai M, Mizukoshi E, Zen Y, Takeshita Y, et al. Histological course of nonalcoholic fatty liver disease in Japanese patients: tight glycemic control, rather than weight reduction, ameliorates liver fibrosis. Diabetes Care. 2010; 33:284–6.18. Alexopoulos AS, Crowley MJ, Wang Y, Moylan CA, Guy CD, Henao R, et al. Glycemic control predicts severity of hepatocyte ballooning and hepatic fibrosis in nonalcoholic fatty liver disease. Hepatology. 2021; 74:1220–33.
Article19. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005; 41:1313–21.
Article20. Farrell GC, McCullough AJ, Day CP. Non-alcoholic fatty liver disease: a practical guide. Hoboken: Wiley-Blackwell;2013. Chapter 3, Pathology of NAFLD. p. 27–36.21. Konishi K, Miyake T, Furukawa S, Senba H, Kanzaki S, Nakaguchi H, et al. Advanced fibrosis of non-alcoholic steatohepatitis affects the significance of lipoprotein(a) as a cardiovascular risk factor. Atherosclerosis. 2020; 299:32–37.
Article22. Miyake T, Furukawa S, Matsuura B, Yoshida O, Miyazaki M, Shiomi A, et al. Plasma fatty acid composition is associated with histological findings of nonalcoholic steatohepatitis. Biomedicines. 2022; 10:2540.
Article23. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. 2021; 44(Suppl 1):S15–33.24. Leite NC, Villela-Nogueira CA, Pannain VL, Bottino AC, Rezende GF, Cardoso CR, et al. Histopathological stages of nonalcoholic fatty liver disease in type 2 diabetes: prevalences and correlated factors. Liver Int. 2011; 31:700–6.
Article25. Moller JB, Dalla Man C, Overgaard RV, Ingwersen SH, Tornoe CW, Pedersen M, et al. Ethnic differences in insulin sensitivity, β-cell function, and hepatic extraction between Japanese and Caucasians: a minimal model analysis. J Clin Endocrinol Metab. 2014; 99:4273–80.
Article26. Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999; 107:450–5.
Article27. Arrese M, Cabrera D, Kalergis AM, Feldstein AE. Innate immunity and inflammation in NAFLD/NASH. Dig Dis Sci. 2016; 61:1294–303.
Article28. Rolo AP, Teodoro JS, Palmeira CM. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic Biol Med. 2012; 52:59–69.
Article29. Lake AD, Novak P, Hardwick RN, Flores-Keown B, Zhao F, Klimecki WT, et al. The adaptive endoplasmic reticulum stress response to lipotoxicity in progressive human nonalcoholic fatty liver disease. Toxicol Sci. 2014; 137:26–35.
Article30. DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988; 37:667–87.31. Addepally NS, George N, Martinez-Macias R, Garcia-Saenz-de-Sicilia M, Kim WR, Duarte-Rojo A. Hemoglobin A1c has suboptimal performance to diagnose and monitor diabetes mellitus in patients with cirrhosis. Dig Dis Sci. 2018; 63:3498–508.
Article32. Miyake T, Kumagi T, Hirooka M, Furukawa S, Koizumi M, Tokumoto Y, et al. Body mass index is the most useful predictive factor for the onset of nonalcoholic fatty liver disease: a community-based retrospective longitudinal cohort study. J Gastroenterol. 2013; 48:413–22.
Article33. Miyake T, Kumagi T, Furukawa S, Tokumoto Y, Hirooka M, Abe M, et al. Non-alcoholic fatty liver disease: factors associated with its presence and onset. J Gastroenterol Hepatol. 2013; 28 Suppl 4:71–8.
Article34. Tilg H, Hotamisligil GS. Nonalcoholic fatty liver disease: cytokine-adipokine interplay and regulation of insulin resistance. Gastroenterology. 2006; 131:934–45.
Article35. Tominaga M, Eguchi H, Manaka H, Igarashi K, Kato T, Sekikawa A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care. 1999; 22:920–4.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Diagnosis of Nonalcoholic Fatty Liver Disease
- Noninvasive serum biomarkers for liver steatosis in nonalcoholic fatty liver disease: Current and future developments
- Nonalcoholic fatty liver disease: pathogenesis and treatment
- Diagnostic Assessment of Nonalcoholic Fatty Liver Disease
- Elevated serum bilirubin levels are inversely associated with nonalcoholic fatty liver disease