J Korean Assoc Oral Maxillofac Surg.
2024 Apr;50(2):103-109. 10.5125/jkaoms.2024.50.2.103.
Adjunctive recombinant human parathyroid hormone agents for the treatment of medication-related osteonecrosis of the jaw: a report of three cases
- Affiliations
-
- 1Department of Oral and Maxillofacial Surgery, Ewha Womans University Medical Center, Ewha Womans University Mokdong Hospital, Seoul, Korea
- 2Department of Oral and Maxillofacial Surgery, Ewha Womans University Medical Center, Ewha Womans University Seoul Hospital, Seoul, Korea
- KMID: 2555740
- DOI: http://doi.org/10.5125/jkaoms.2024.50.2.103
Abstract
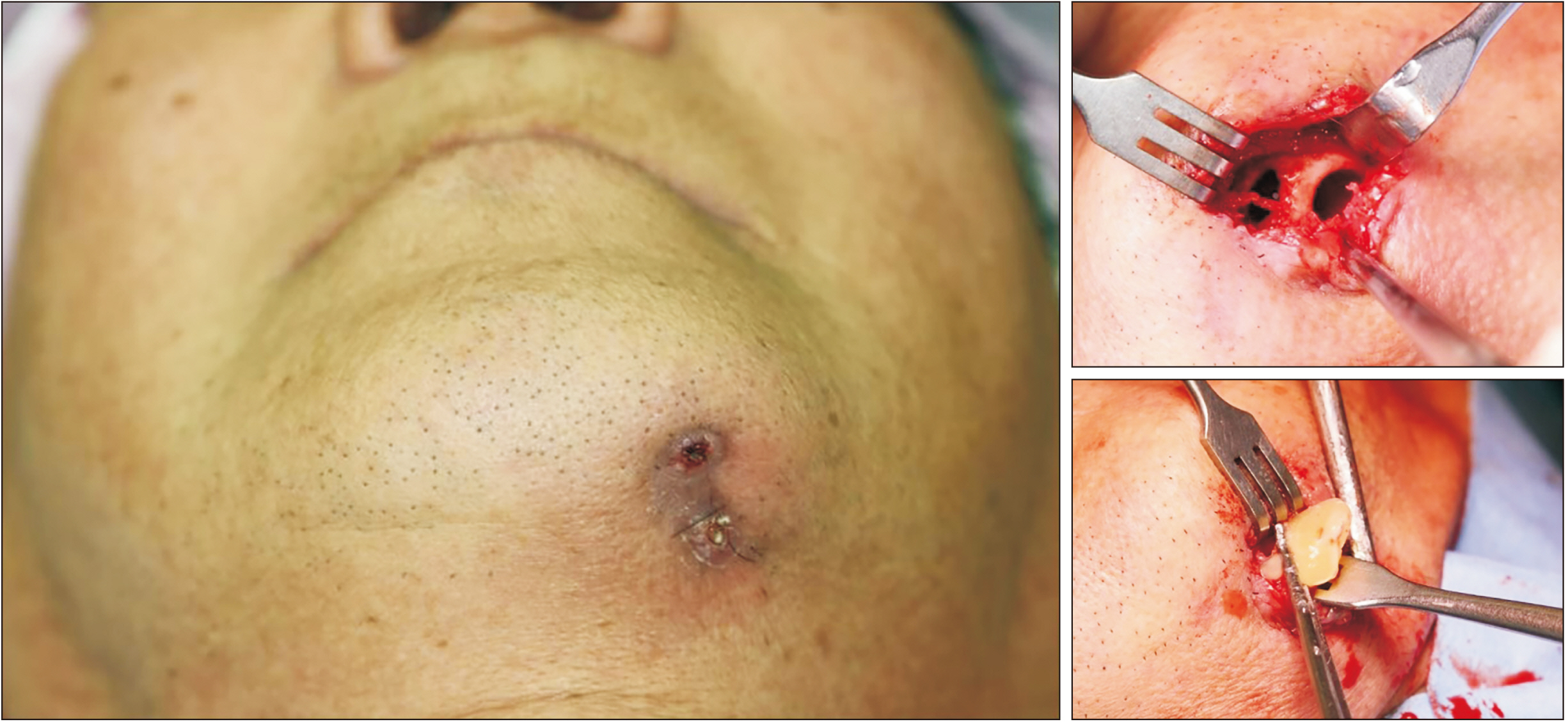
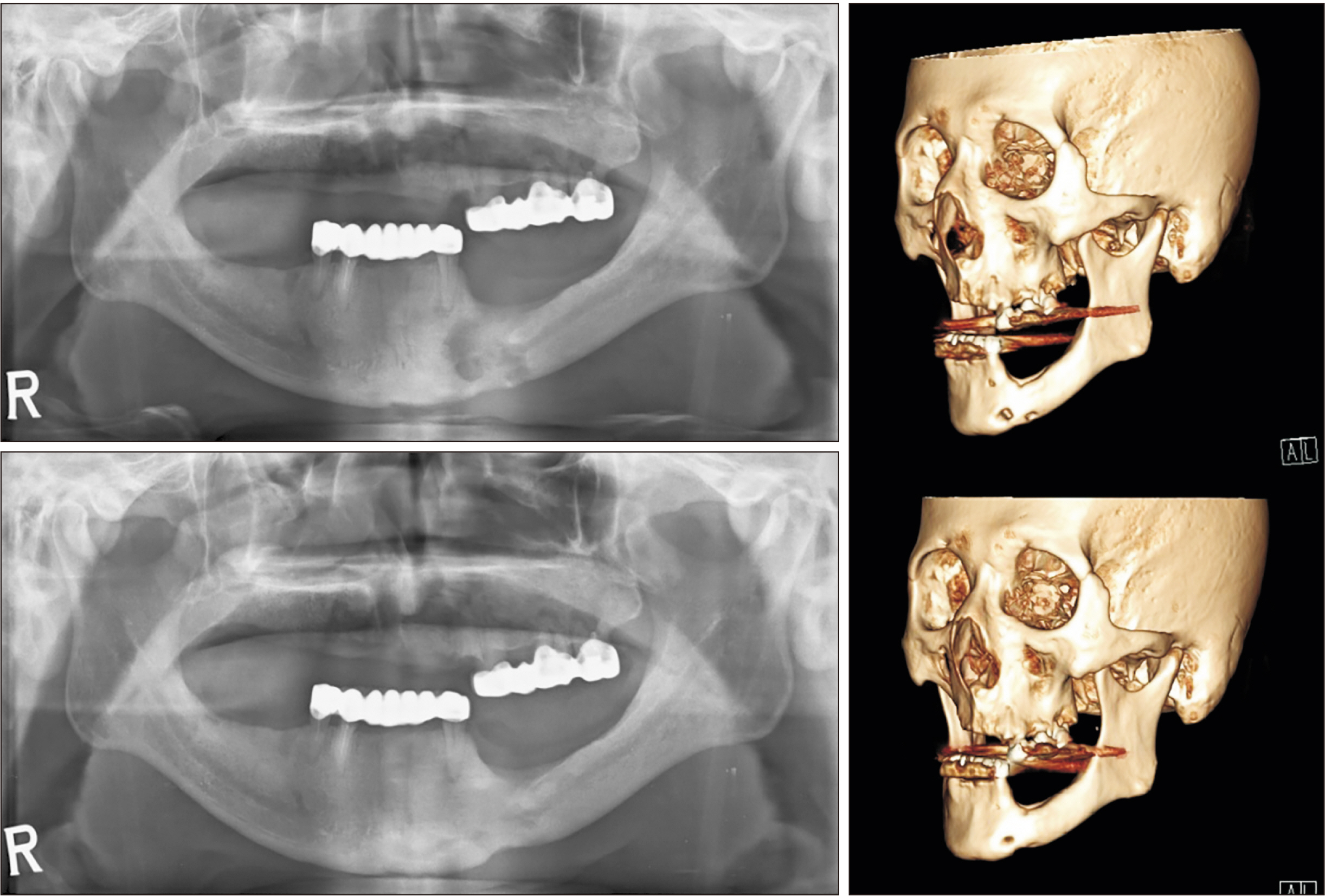
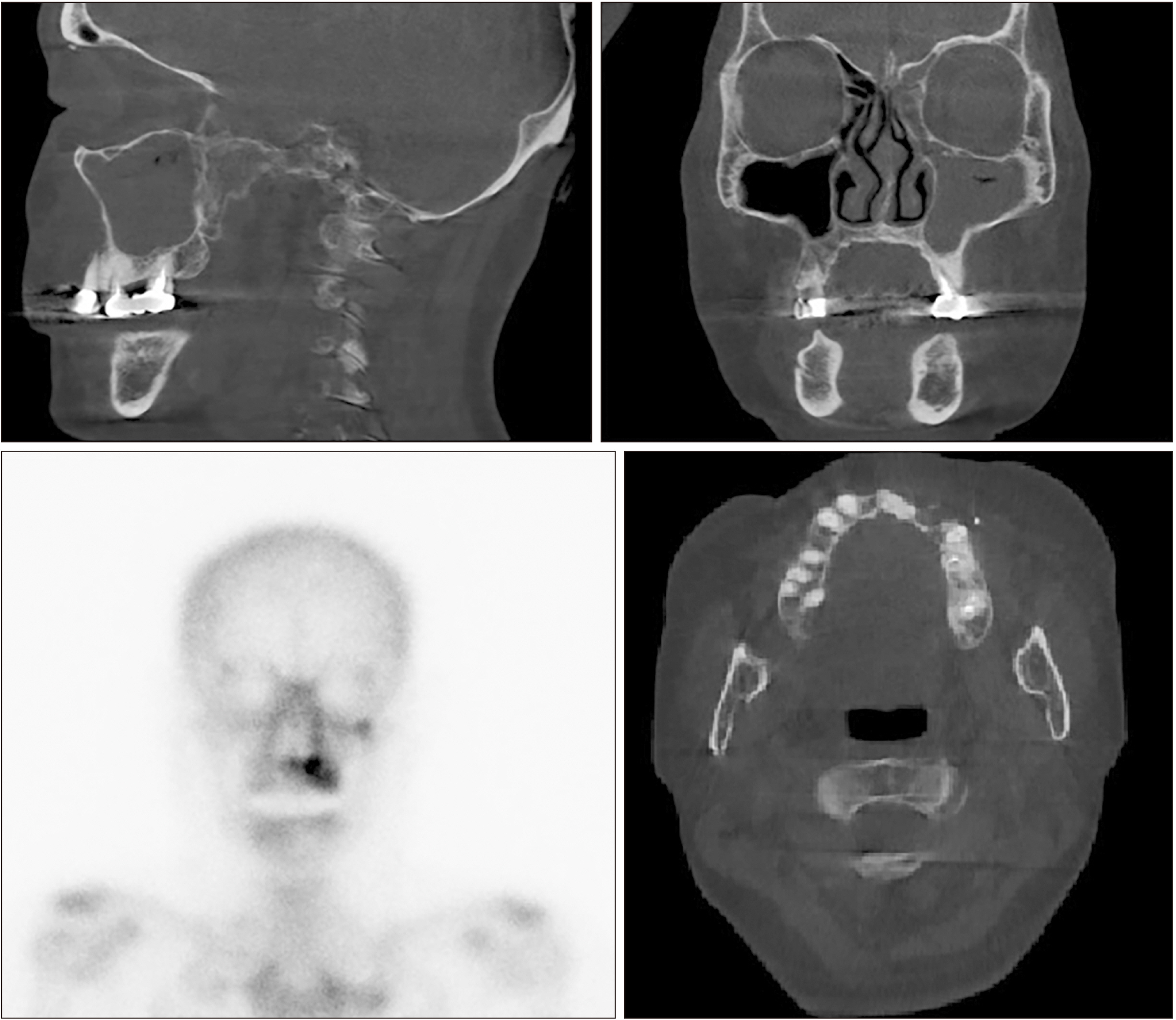
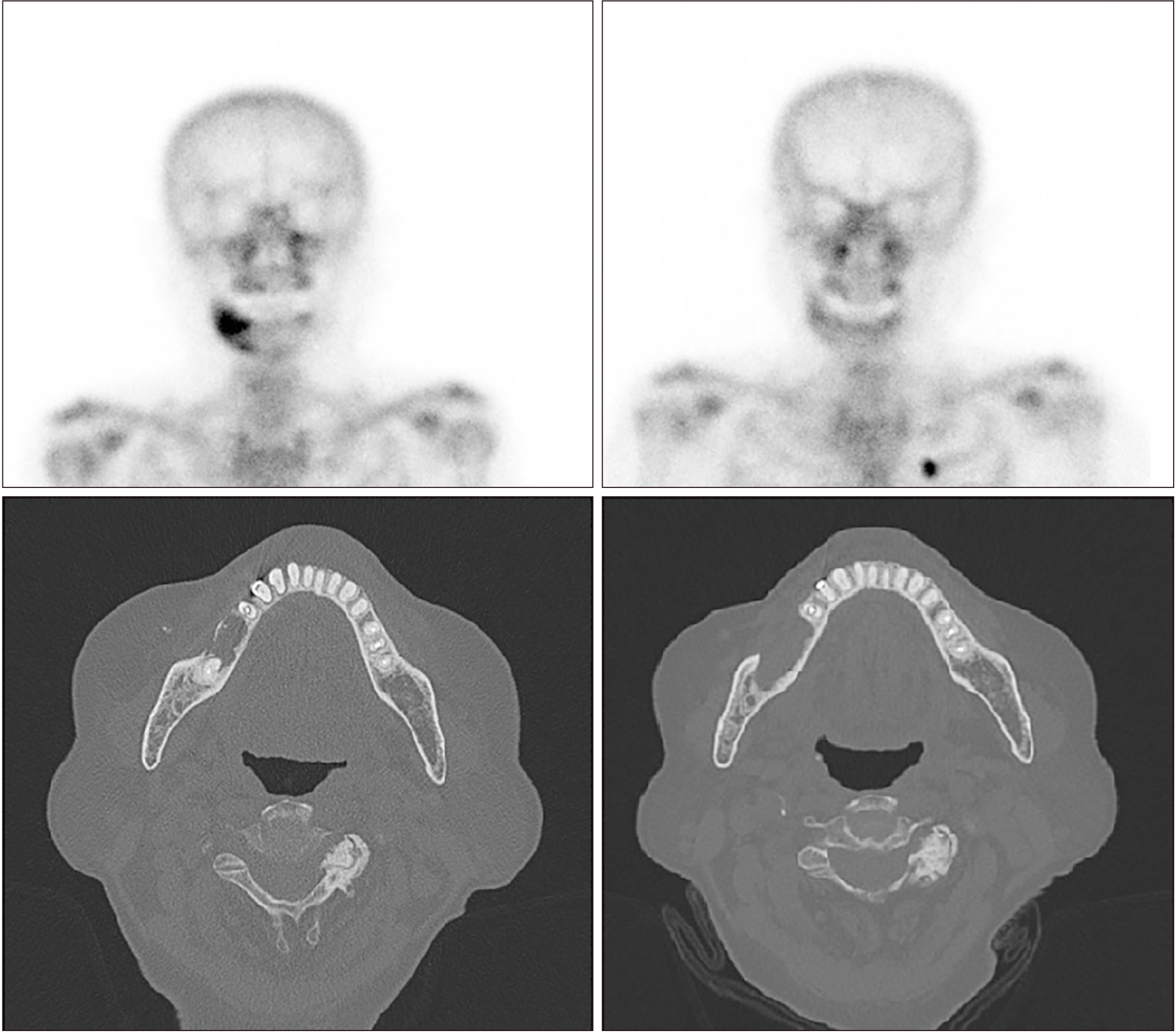
- Teriparatide has been effective in treating people diagnosed with medication-related osteonecrosis of the jaw (MRONJ). However, its efficacy is not well established to be accepted as a standard of care. The objective of this paper was to investigate the efficacy of recombinant human parathyroid hormone for the treatment of MRONJ. We report three cases of MRONJ patients with osteoporosis as the primary disease who were treated with a teriparatide agent along with other adjunctive measures. Each patient was administered a teriparatide injection subcutaneously for 16 weeks, 36 weeks, or 60 weeks. Surgical intervention including partial resection, sequestrectomy, decortication, and saucerization took place during the teriparatide administration. Complete lesion resolution was identified clinically and radiographically in all three patients. In patients diagnosed with MRONJ, teriparatide therapy is an efficacious and safe therapeutic option to improve healing of bone lesions. These findings demonstrate that teriparatide in combination with another therapy, especially bone morphogenetic protein, platelet-rich fibrin, or antibiotic therapy, can be an effective protocol for MRONJ.
Keyword
Figure
Cited by 1 articles
-
Successful treatment of adjunctive teriparatide therapy for medication-related osteonecrosis of the jaw: a report of two cases
Ra-yeon Kim, Sung ok Hong, Jae-woong Jung, Mu-hang Lee, Young-kee Lee, Yu-jin Jee
J Korean Assoc Oral Maxillofac Surg. 2024;50(5):285-291. doi: 10.5125/jkaoms.2024.50.5.285.
Reference
-
References
1. Sim IW, Borromeo GL, Tsao C, Hardiman R, Hofman MS, Papatziamos Hjelle C, et al. 2020; Teriparatide promotes bone healing in medication-related osteonecrosis of the jaw: a placebo-controlled, randomized trial. J Clin Oncol. 38:2971–80. https://doi.org/10.1200/jco.19.02192. DOI: 10.1200/JCO.19.02192. PMID: 32614699.
Article2. Khan AA, Morrison A, Kendler DL, Rizzoli R, Hanley DA, Felsenberg D, et al. 2017; ; International Task Force on Osteonecrosis of the Jaw. Case-based review of osteonecrosis of the jaw (ONJ) and application of the international recommendations for management from the International Task Force on ONJ. J Clin Densitom. 20:8–24. https://doi.org/10.1016/j.jocd.2016.09.005. DOI: 10.1016/j.jocd.2016.09.005. PMID: 27956123.
Article3. Rubin MR, Bilezikian JP. 2003; The anabolic effects of parathyroid hormone therapy. Clin Geriatr Med. 19:415–32. https://doi.org/10.1016/s0749-0690(02)00074-5. DOI: 10.1016/S0749-0690(02)00074-5. PMID: 12916294.
Article4. Eastell R, Walsh JS. 2017; Anabolic treatment for osteoporosis: teriparatide. Clin Cases Miner Bone Metab. 14:173–8. https://doi.org/10.11138/ccmbm/2017.14.1.173. DOI: 10.11138/ccmbm/2017.14.1.173. PMID: 29263728. PMCID: PMC5726204.
Article5. Dempster DW, Cosman F, Parisien M, Shen V, Lindsay R. 1993; Anabolic actions of parathyroid hormone on bone. Endocr Rev. 14:690–709. https://doi.org/10.1210/edrv-14-6-690. DOI: 10.1210/edrv-14-6-690. PMID: 8119233.
Article6. Frost ML, Siddique M, Blake GM, Moore AE, Schleyer PJ, Dunn JT, et al. 2011; Differential effects of teriparatide on regional bone formation using (18)F-fluoride positron emission tomography. J Bone Miner Res. 26:1002–11. https://doi.org/10.1002/jbmr.305. DOI: 10.1002/jbmr.305. PMID: 21542003.
Article7. Harper RP, Fung E. 2007; Resolution of bisphosphonate-associated osteonecrosis of the mandible: possible application for intermittent low-dose parathyroid hormone [rhPTH(1-34)]. J Oral Maxillofac Surg. 65:573–80. https://doi.org/10.1016/j.joms.2006.10.076. DOI: 10.1016/j.joms.2006.10.076. PMID: 17307613.
Article8. Narongroeknawin P, Danila MI, Humphreys LG Jr, Barasch A, Curtis JR. 2010; Bisphosphonate-associated osteonecrosis of the jaw, with healing after teriparatide: a review of the literature and a case report. Spec Care Dentist. 30:77–82. https://doi.org/10.1111/j.1754-4505.2009.00128.x. DOI: 10.1111/j.1754-4505.2009.00128.x. PMID: 20415805. PMCID: PMC4097110.
Article9. Iwamoto J, Yago K, Sato Y, Matsumoto H. 2012; Teriparatide therapy for bisphosphonate-associated osteonecrosis of the jaw in an elderly Japanese woman with severe osteoporosis. Clin Drug Investig. 32:547–53. https://doi.org/10.1007/bf03261908. DOI: 10.1007/BF03261908. PMID: 22734599.
Article10. Kim KM, Park W, Oh SY, Kim HJ, Nam W, Lim SK, et al. 2014; Distinctive role of 6-month teriparatide treatment on intractable bisphosphonate-related osteonecrosis of the jaw. Osteoporos Int. 25:1625–32. https://doi.org/10.1007/s00198-014-2622-8. DOI: 10.1007/s00198-014-2622-8. PMID: 24554340.
Article11. Kakehashi H, Ando T, Minamizato T, Nakatani Y, Kawasaki T, Ikeda H, et al. 2015; Administration of teriparatide improves the symptoms of advanced bisphosphonate-related osteonecrosis of the jaw: preliminary findings. Int J Oral Maxillofac Surg. 44:1558–64. https://doi.org/10.1016/j.ijom.2015.07.018. DOI: 10.1016/j.ijom.2015.07.018. PMID: 26304604.
Article12. Dos Santos Ferreira L, Abreu LG, Calderipe CB, Martins MD, Schuch LF, Vasconcelos ACU. 2021; Is teriparatide therapy effective for medication-related osteonecrosis of the jaw? A systematic review and meta-analysis. Osteoporos Int. 32:2449–59. https://doi.org/10.1007/s00198-021-06078-z. DOI: 10.1007/s00198-021-06078-z. PMID: 34331067.
Article13. Eli Lilly and Company. 2024. FORTEO (teriparatide [rDNA origin] injection) for subcutaneous use [Internet]. U.S. Food and Drug Administration;Silver Spring (MD): Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021318s012lbl.pdf. cited 2024 Feb 7.14. Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. 2001; Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 344:1434–41. https://doi.org/10.1056/nejm200105103441904. DOI: 10.1056/NEJM200105103441904. PMID: 11346808.
Article15. Morishita K, Yamada SI, Kawakita A, Hashidume M, Tachibana A, Takeuchi N, et al. 2020; Treatment outcomes of adjunctive teriparatide therapy for medication-related osteonecrosis of the jaw (MRONJ): a multicenter retrospective analysis in Japan. J Orthop Sci. 25:1079–83. https://doi.org/10.1016/j.jos.2020.01.012. DOI: 10.1016/j.jos.2020.01.012. PMID: 32111549.
Article16. Krege JH, Gilsenan AW, Komacko JL, Kellier-Steele N. 2022; Teriparatide and osteosarcoma risk: history, science, elimination of boxed warning, and other label updates. JBMR Plus. 6:e10665. https://doi.org/10.1002/jbm4.10665. DOI: 10.1002/jbm4.10665. PMID: 36111201. PMCID: PMC9465003.
Article17. Carlson ER, Basile JD. 2009; The role of surgical resection in the management of bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 67(5 Suppl):85–95. https://doi.org/10.1016/j.joms.2009.01.006. DOI: 10.1016/j.joms.2009.01.006. PMID: 19371819.
Article18. Stockmann P, Burger M, von Wilmowsky C, Ebker T, Lutz R, Bauersachs A, et al. 2014; The outcome after surgical therapy of bisphosphonate-associated osteonecrosis of the jaw--results of a clinical case series with an average follow-up of 20 months. Clin Oral Investig. 18:1299–304. https://doi.org/10.1007/s00784-013-1092-2. DOI: 10.1007/s00784-013-1092-2. PMID: 23989467.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Successful treatment of adjunctive teriparatide therapy for medicationrelated osteonecrosis of the jaw: a report of two cases
- Teriparatide therapy without surgical treatment for medication-related osteonecrosis of the jaw: a report of two cases
- Medication-Related Osteonecrosis of the Jaws: A Literature Review
- A critical assessment of the medication-related osteonecrosis of the jaw classification in stage I patients: a retrospective analysis
- Bisphosphonate-Related Osteonecrosis in a Patient with Florid Cemento-Osseous Dysplasia