J Korean Assoc Oral Maxillofac Surg.
2024 Apr;50(2):86-93. 10.5125/jkaoms.2024.50.2.86.
Effect of tranexamic acid on blood loss reduction in patients undergoing orthognathic surgery under hypotensive anesthesia: a single-center, retrospective, observational study
- Affiliations
-
- 1Department of Oral and Maxillofacial Surgery, Shimane University Faculty of Medicine, Izumo, Japan
- 2Department of Anesthesiology, Saitama Medical University, Saitama, Japan
- 3Department of Anesthesiology, Shimane University Faculty of Medicine, Izumo, Japan
- 4Department of Palliative Care, Shimane University Faculty of Medicine, Izumo, Japan
- KMID: 2555738
- DOI: http://doi.org/10.5125/jkaoms.2024.50.2.86
Abstract
Objectives
Orthognathic surgery is a surgical procedure performed by intraoral approach with established and safe techniques; however, excessive blood loss has been reported in rare cases. In response, investigative efforts to identify methods to reduce the amount of blood loss have been made. Among such methods, the administration of tranexamic acid was reported to reduce the amount of intraoperative blood loss. However, few studies to date have reported the effect of tranexamic acid in orthognathic surgery under hypotensive anesthesia. The present study aimed to investigate the effect of the administration of tranexamic acid on intraoperative blood loss in patients undergoing bimaxillary (maxillary and mandibular) orthognathic surgery under hypotensive anesthesia.
Patients and Methods
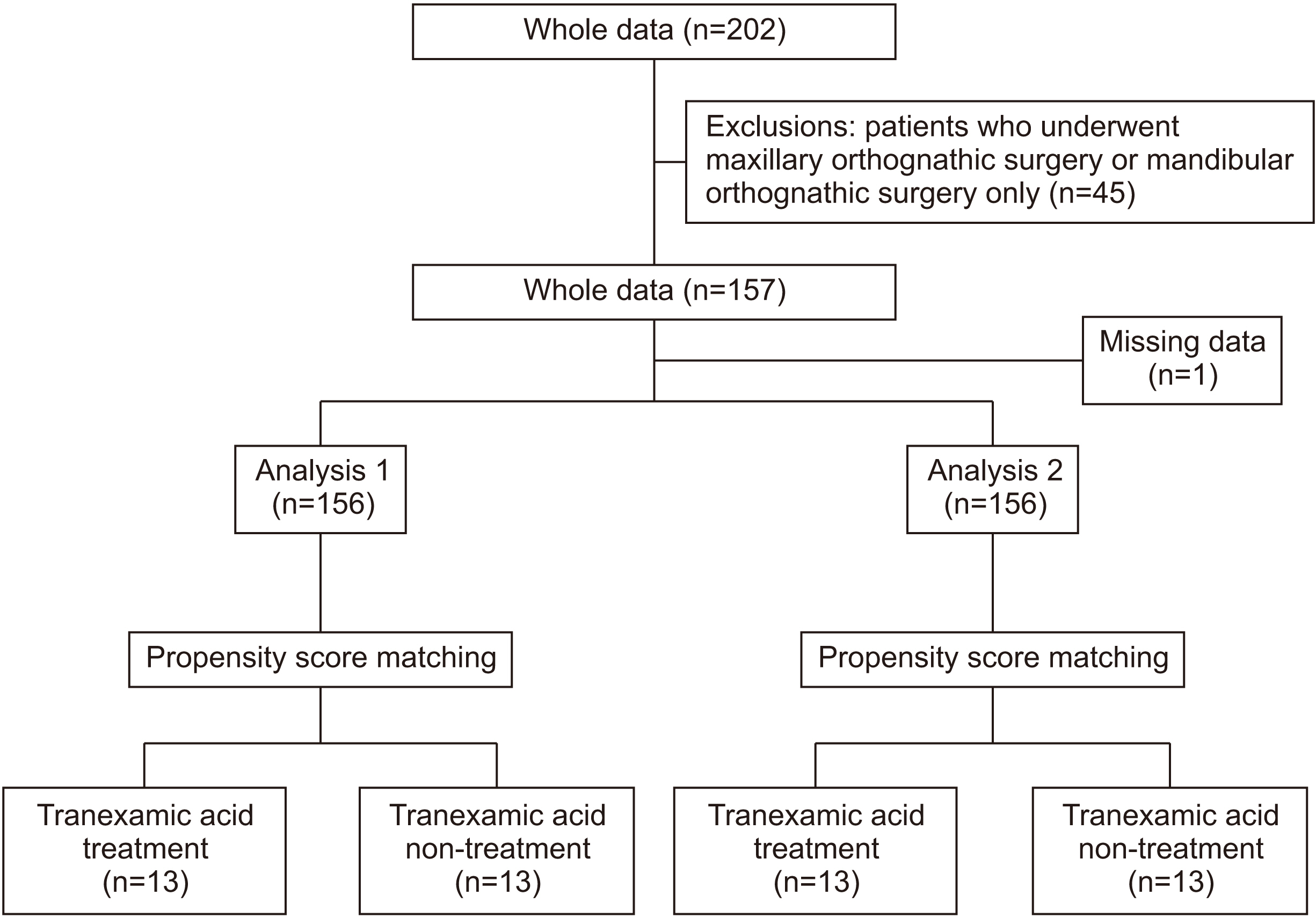
A total of 156 patients (mean age, 27.0±10.8 years) who underwent bimaxillary orthognathic surgery under hypotensive anesthesia performed by the same surgeon between June 2013 and February 2022 were included in this study. The following data were collected from the medical records of each patient: background factors (age, sex, and body mass index), use of tranexamic acid, surgical procedures, previous medical history, duration of surgery, American Society of Anesthesiology physical status findings before surgery, intraoperative blood loss as a primary outcome, in–out balance, and blood test results. Descriptive statistics were calculated for statistical analysis, and a t-test and the chi-squared test were used for between-group comparisons. Group comparisons were performed after 1:1 propensity score matching to adjust for confounding factors. Statistical significance was set at P<0.05.
Results
Comparison between the groups based on the use of tranexamic acid revealed a significant difference in operation time. Propensity score matching analysis revealed that intraoperative blood loss was significantly lower in the tranexamic acid group.
Conclusion
The administration of tranexamic acid was effective in reducing intraoperative blood loss in patients undergoing bimaxillary orthognathic surgery under hypotensive anesthesia.
Figure
Reference
-
References
1. Weiss RO 2nd, Ong AA, Reddy LV, Bahmanyar S, Vincent AG, Ducic Y. 2021; Orthognathic surgery-LeFort I osteotomy. Facial Plast Surg. 37:703–8. https://doi.org/10.1055/s-0041-1735308. DOI: 10.1055/s-0041-1735308. PMID: 34530468.
Article2. Lee KT, Lin SS, Hsu KJ, Tsai CY, Lee YH, Chang YJ, et al. 2021; Intraoperative blood loss and postoperative pain in the sagittal split ramus osteotomy and intraoral vertical ramus osteotomy: a literature review. Biomed Res Int. 2021:4439867. https://doi.org/10.1155/2021/4439867. DOI: 10.1155/2021/4439867. PMID: 34285911. PMCID: PMC8275401.
Article3. Alister Herdener JP, Uribe F, Barreda M, Olate S, Fariña R. 2021; Delayed bleeding of sphenopalatine artery as a complication in Le Fort I osteotomy. J Craniofac Surg. 32:e493–5. https://doi.org/10.1097/scs.0000000000007455. DOI: 10.1097/SCS.0000000000007455. PMID: 33481476.
Article4. Yang HJ, Jung YE, Kwon IJ, Lee JY, Hwang SJ. 2020; Airway changes and prevalence of obstructive sleep apnoea after bimaxillary orthognathic surgery with large mandibular setback. Int J Oral Maxillofac Surg. 49:342–9. https://doi.org/10.1016/j.ijom.2019.07.012. DOI: 10.1016/j.ijom.2019.07.012. PMID: 31451303.
Article5. Remschmidt B, Schwaiger M, Gaessler J, Wallner J, Zemann W, Schwaiger M. 2023; Surgical site infections in orthognathic surgery: prolonged versus single-dose antibiotic prophylaxis. Int J Oral Maxillofac Surg. 52:219–26. https://doi.org/10.1016/j.ijom.2022.06.002. DOI: 10.1016/j.ijom.2022.06.002. PMID: 35760661.
Article6. Dujoncquoy JP, Ferri J, Raoul G, Kleinheinz J. 2010; Temporomandibular joint dysfunction and orthognathic surgery: a retrospective study. Head Face Med. 6:27. https://doi.org/10.1186/1746-160x-6-27. DOI: 10.1186/1746-160X-6-27. PMID: 21083902. PMCID: PMC2998459.
Article7. Ulker O, Demirbas AE, Kutuk N, Kilic E, Alkan A. 2021; Vascular complications in Le Fort I osteotomy: incidence, reasons, and management of the intraoperative hemorrhage. J Craniofac Surg. 32:325–8. https://doi.org/10.1097/scs.0000000000007152. DOI: 10.1097/SCS.0000000000007152. PMID: 33156169.
Article8. Salma RG, Al-Shammari FM, Al-Garni BA, Al-Qarzaee MA. 2017; Operative time, blood loss, hemoglobin drop, blood transfusion, and hospital stay in orthognathic surgery. Oral Maxillofac Surg. 21:259–66. https://doi.org/10.1007/s10006-017-0626-1. DOI: 10.1007/s10006-017-0626-1. PMID: 28466191.
Article9. Piñeiro-Aguilar A, Somoza-Martín M, Gandara-Rey JM, García-García A. 2011; Blood loss in orthognathic surgery: a systematic review. J Oral Maxillofac Surg. 69:885–92. https://doi.org/10.1016/j.joms.2010.07.019. DOI: 10.1016/j.joms.2010.07.019. PMID: 21195531.
Article10. Lin S, McKenna SJ, Yao CF, Chen YR, Chen C. 2017; Effects of hypotensive anesthesia on reducing intraoperative blood loss, duration of operation, and quality of surgical field during orthognathic surgery: a systematic review and meta-analysis of randomized controlled trials. J Oral Maxillofac Surg. 75:73–86. https://doi.org/10.1016/j.joms.2016.07.012. DOI: 10.1016/j.joms.2016.07.012. PMID: 27542543.
Article11. Gallagher DM, Milliken RA. 1979; Induced hypotension for orthognathic surgery. J Oral Surg. 37:47–51.12. Elsharnouby NM, Elsharnouby MM. 2006; Magnesium sulphate as a technique of hypotensive anaesthesia. Br J Anaesth. 96:727–31. https://doi.org/10.1093/bja/ael085. DOI: 10.1093/bja/ael085. PMID: 16670112.
Article13. Jiang J, Zhou R, Li B, Xue F. 2019; Is deliberate hypotension a safe technique for orthopedic surgery?: a systematic review and meta-analysis of parallel randomized controlled trials. J Orthop Surg Res. 14:409. https://doi.org/10.1186/s13018-019-1473-6. DOI: 10.1186/s13018-019-1473-6. PMID: 31791362. PMCID: PMC6889611.
Article14. Dutton RP. 2004; Controlled hypotension for spinal surgery. Eur Spine J. 13(Suppl 1):S66–71. https://doi.org/10.1007/s00586-004-0756-7. DOI: 10.1007/s00586-004-0756-7. PMID: 15197633. PMCID: PMC3592182.
Article15. Zhang Q, Yin S, Huang K, Wang M, Xie H, Liao R, et al. 2021; [Effectiveness and safety of tranexamic acid combined with intraoperative controlled hypotension on reducing perioperative blood loss in primary total hip arthroplasty]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 35:1133–40. Chinese. https://doi.org/10.7507/1002-1892.202103230.
Article16. Jozefowicz E, Sabourdin N, Lambelin V, Lejeune V, Delassus R, Tavernier B. 2022; The effect of tranexamic acid on blood loss in orthognathic surgery: a randomized, placebo-controlled, equivalence study. Int J Oral Maxillofac Surg. 51:637–42. https://doi.org/10.1016/j.ijom.2021.08.018. DOI: 10.1016/j.ijom.2021.08.018. PMID: 34465477.
Article17. Williams-Johnson JA, McDonald AH, Strachan GG, Williams EW. 2010; Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. West Indian Med J. 59:612–24.18. Berebichez-Fridman R, Castillo-Vázquez FG, Berebichez-Fastlicht E. 2021; [Applications of tranexamic acid in orthopaedics and traumatology]. Acta Ortop Mex. 35:539–46. Spanish. DOI: 10.35366/105708.19. Alaifan T, Alenazy A, Xiang Wang D, Fernando SM, Spence J, Belley-Cote E, et al. 2019; Tranexamic acid in cardiac surgery: a systematic review and meta-analysis (protocol). BMJ Open. 9:e028585. https://doi.org/10.1136/bmjopen-2018-028585. DOI: 10.1136/bmjopen-2018-028585. PMID: 31530593. PMCID: PMC6756438.
Article20. Dunn CJ, Goa KL. 1999; Tranexamic acid: a review of its use in surgery and other indications. Drugs. 57:1005–32. https://doi.org/10.2165/00003495-199957060-00017. DOI: 10.2165/00003495-199957060-00017. PMID: 10400410.
Article21. Sundström A, Seaman H, Kieler H, Alfredsson L. 2009; The risk of venous thromboembolism associated with the use of tranexamic acid and other drugs used to treat menorrhagia: a case-control study using the General Practice Research Database. BJOG. 116:91–7. https://doi.org/10.1111/j.1471-0528.2008.01926.x. DOI: 10.1111/j.1471-0528.2008.01926.x. PMID: 19016686.
Article22. Kim DH, Kim S, Kang H, Jin HJ, Hwang SH. 2019; Efficacy of tranexamic acid on operative bleeding in endoscopic sinus surgery: a meta-analysis and systematic review. Laryngoscope. 129:800–7. https://doi.org/10.1002/lary.27766. DOI: 10.1002/lary.27766. PMID: 30593688.
Article23. Murphy WG, Davies MJ, Eduardo A. 1993; The haemostatic response to surgery and trauma. Br J Anaesth. 70:205–13. https://doi.org/10.1093/bja/70.2.205. DOI: 10.1093/bja/70.2.205. PMID: 7679584.
Article24. Levin EG, Marzec U, Anderson J, Harker LA. 1984; Thrombin stimulates tissue plasminogen activator release from cultured human endothelial cells. J Clin Invest. 74:1988–95. https://doi.org/10.1172/jci111620. DOI: 10.1172/JCI111620. PMID: 6542570. PMCID: PMC425386.
Article25. Hoylaerts M, Lijnen HR, Collen D. 1981; Studies on the mechanism of the antifibrinolytic action of tranexamic acid. Biochim Biophys Acta. 673:75–85. DOI: 10.1016/0304-4165(81)90312-3. PMID: 7193484.
Article26. Ping WD, Zhao QM, Sun HF, Lu HS, Li F. 2019; Role of tranexamic acid in nasal surgery: a systemic review and meta-analysis of randomized control trial. Medicine (Baltimore). 98:e15202. https://doi.org/10.1097/md.0000000000015202. DOI: 10.1097/MD.0000000000015202. PMID: 31008946. PMCID: PMC6494350.
Article27. Pereira EG, Carvalho MM, Oliveira T, Sacramento T, Cruz H, Viegas R, et al. 2023; Benefits of tranexamic acid in total knee arthroplasty: a classification and regression tree analysis in function of instrumentation, BMI, and gender. J Knee Surg. 36:173–80. https://doi.org/10.1055/s-0041-1731455. DOI: 10.1055/s-0041-1731455. PMID: 34225366.
Article28. Andersson L, Nilsoon IM, Colleen S, Granstrand B, Melander B. 1968; Role of urokinase and tissue activator in sustaining bleeding and the management thereof with EACA and AMCA. Ann N Y Acad Sci. 146:642–58. https://doi.org/10.1111/j.1749-6632.1968.tb20322.x. DOI: 10.1111/j.1749-6632.1968.tb20322.x. PMID: 5254275.
Article29. Nilsson IM. 1980; Clinical pharmacology of aminocaproic and tranexamic acids. J Clin Pathol Suppl (R Coll Pathol). 14:41–7. DOI: 10.1136/jcp.33.Suppl_14.41. PMID: 7000846.
Article30. Niego B, Horvath A, Coughlin PB, Pugsley MK, Medcalf RL. 2008; Desmoteplase-mediated plasminogen activation and clot lysis are inhibited by the lysine analogue tranexamic acid. Blood Coagul Fibrinolysis. 19:322–4. https://doi.org/10.1097/mbc.0b013e3282f54568. DOI: 10.1097/MBC.0b013e3282f54568. PMID: 18469556.
Article31. Benoni G, Lethagen S, Fredin H. 1997; The effect of tranexamic acid on local and plasma fibrinolysis during total knee arthroplasty. Thromb Res. 85:195–206. https://doi.org/10.1016/s0049-3848(97)00004-2. DOI: 10.1016/S0049-3848(97)00004-2. PMID: 9058494.
Article32. Ker K, Prieto-Merino D, Roberts I. 2013; Systematic review, meta-analysis and meta-regression of the effect of tranexamic acid on surgical blood loss. Br J Surg. 100:1271–9. https://doi.org/10.1002/bjs.9193. DOI: 10.1002/bjs.9193. PMID: 23839785.
Article33. Levi M, ten Cate JW. 1995; [Antifibrinolytic therapy]. Ned Tijdschr Geneeskd. 139:613–7. Dutch.34. Siotou K, Siotos C, Azizi A, Cheah MA, Seal SM, Redett RJ, et al. 2019; The role of antifibrinolytics in reducing blood loss during craniofacial or orthognathic surgical procedures: a meta-analysis. J Oral Maxillofac Surg. 77:1245–60. https://doi.org/10.1016/j.joms.2019.01.032. DOI: 10.1016/j.joms.2019.01.032. PMID: 30796910.
Article35. Tarazi JM, Zois TP, Bohm A, Mont MA, Scuderi GR. 2022; Elevated pre-operative D-dimer levels do not impact the effect of tranexamic acid on revision total knee arthroplasty. Orthop Clin North Am. 53:139–43. https://doi.org/10.1016/j.ocl.2021.12.001. DOI: 10.1016/j.ocl.2021.12.001. PMID: 35365258.
Article36. Wright GP, Wolf AM, Waldherr TL, Ritz-Holland D, Laney ED, Chapman HA, et al. 2020; Preoperative tranexamic acid does not reduce transfusion rates in major oncologic surgery: results of a randomized, double-blind, and placebo-controlled trial. J Surg Oncol. 122:1037–42. https://doi.org/10.1002/jso.26142. DOI: 10.1002/jso.26142. PMID: 32737893.
Article37. Heyns M, Knight P, Steve AK, Yeung JK. 2021; A single preoperative dose of tranexamic acid reduces perioperative blood loss: a meta-analysis. Ann Surg. 273:75–81. https://doi.org/10.1097/sla.0000000000003793. DOI: 10.1097/SLA.0000000000003793. PMID: 32224739.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tranexamic Acid Versus a Placebo in Decreasing Blood Loss in Patients Undergoing Spine Surgery
- Effectiveness of hemocoagulase, tranexamic acid, and their combination for reducing blood loss in bimaxillary orthognathic surgery: a retrospective study
- Effectiveness of Tranexamic Acid in Reducing Blood Loss after Spinal Fusion
- Effect of Tranexamic Acid on Blood Loss and Blood Transfusion Reduction after Total Knee Arthroplasty
- Efficacy of Tranexamic Acid during Primary Total Knee Arthroplasty: Comparative Study between Intravenous Use and Topical Use