Ann Surg Treat Res.
2024 May;106(5):274-283. 10.4174/astr.2024.106.5.274.
Epigenetic modulation inhibits epithelial-mesenchymal transition-driven fibrogenesis and enhances characteristics of chemically-derived hepatic progenitors
- Affiliations
-
- 1Department of Surgery, Hanyang University College of Medicine, Seoul, Korea
- 2Research Institute of Regenerative Medicine and Stem Cells, Hanyang University, Seoul, Korea
- 3Major in Medical Genetics, Department of Medicine, Graduate School, Hanyang University, Seoul, Korea
- 4Department of Genetics, Hanyang University College of Medicine, Seoul, Korea
- 5Department of Biomedical Science, Graduate School of Biomedical Science and Engineering, Hanyang University, Seoul, Korea
- KMID: 2555724
- DOI: http://doi.org/10.4174/astr.2024.106.5.274
Abstract
- Purpose
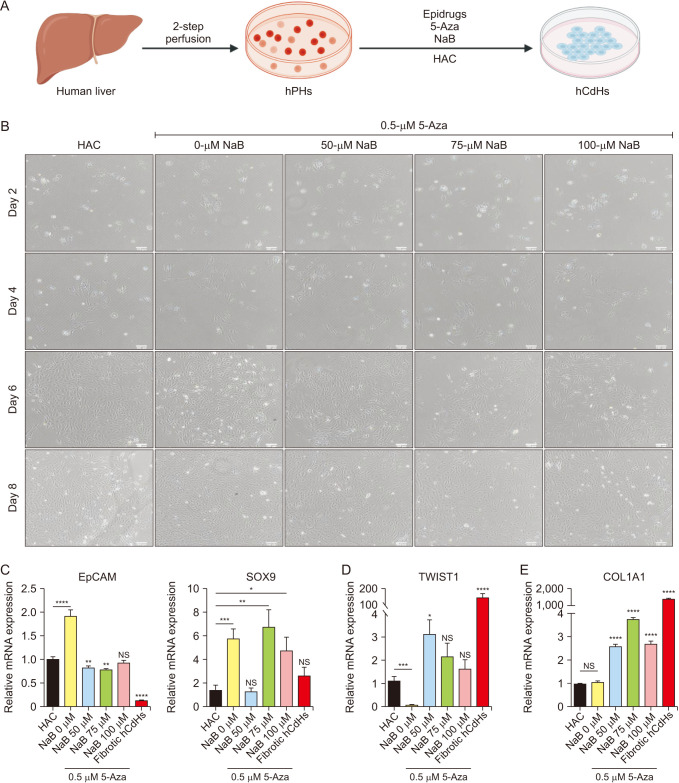
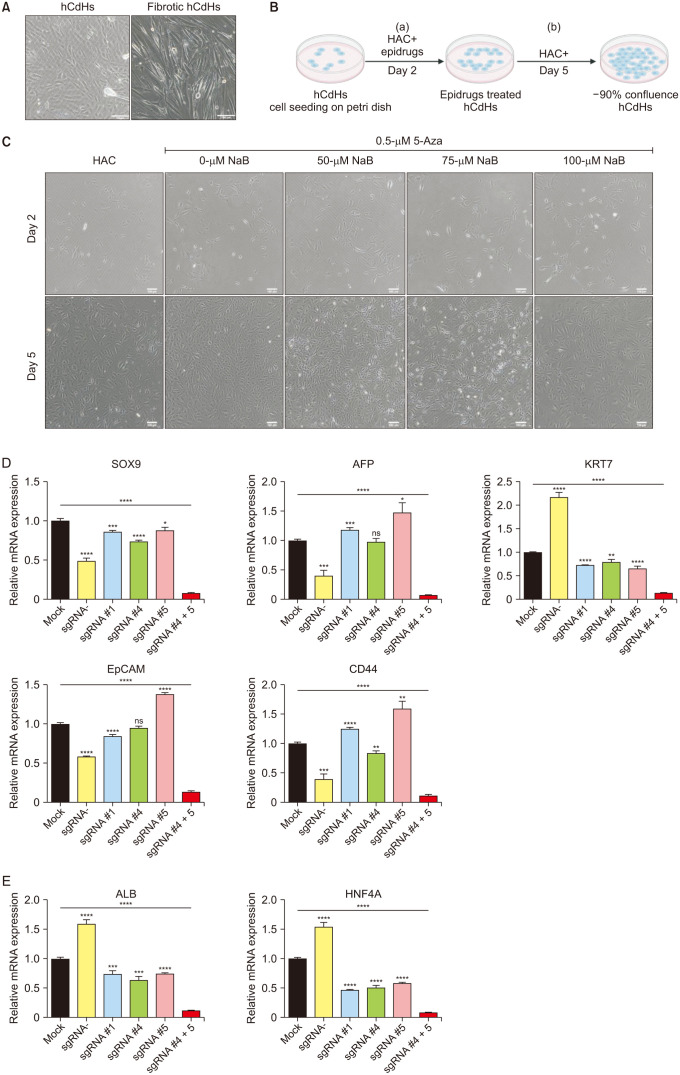
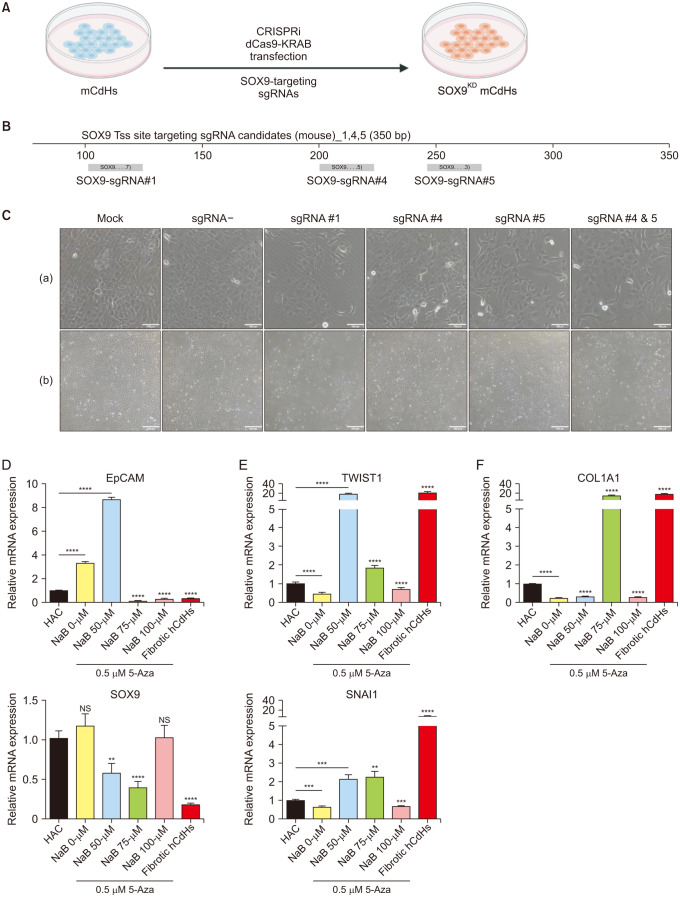
One of the novel cell sources of cell-based liver regenerative medicine is human chemically-derived hepatic progenitors (hCdHs). We previously established this cell by direct hepatocyte reprogramming with a combination of small molecules (hepatocyte growth factor, A83-01, CHIR99021). However, there have been several issues concerning the cell’s stability and maintenance, namely the occurrences of epithelial-mesenchymal transition (EMT) that develop fibrotic phenotypes, resulting in the loss of hepatic progenitor characteristics. These hepatic progenitor attributes are thought to be regulated by SOX9, a transcription factor essential for hepatic progenitor cells and cholangiocytes.
Methods
To suppress the fibrotic phenotype and improve our long-term hCdHs culture technology, we utilized the epigenetic modulating drugs DNA methyltransferase inhibitor (5-azacytidine) and histone deacetylase inhibitor (sodium butyrate) that have been reported to suppress and revert hepatic fibrosis. To confirm the essential role of SOX9 to our cell, we used clustered regularly interspaced short palindromic repeats-interference (CRISPRi) to repress the SOX9 expression.
Results
The treatment of only 5-azacytidine significantly reduces the fibrosis/mesenchymal marker and EMT-related transcription factor expression level in the early passages. Interestingly, this treatment also increased the hepatic progenitor markers expression, even during the reprogramming phase. Then, we confirmed the essential role of SOX9 by repressing the SOX9 expression with CRISPRi which resulted in the downregulation of several essential hepatic progenitor cell markers.
Conclusion
These results highlight the capacity of 5-azacytidine to inhibit EMT-driven hepatic fibrosis and the significance of SOX9 on hepatic progenitor cell stemness properties.
Figure
Reference
-
1. Oh SH, Swiderska-Syn M, Jewell ML, Premont RT, Diehl AM. Liver regeneration requires Yap1-TGFβ-dependent epithelial-mesenchymal transition in hepatocytes. J Hepatol. 2018; 69:359–367. PMID: 29758331.2. Kim Y, Kang K, Lee SB, Seo D, Yoon S, Kim SJ, et al. Small molecule-mediated reprogramming of human hepatocytes into bipotent progenitor cells. J Hepatol. 2019; 70:97–107. PMID: 30240598.3. Thorgersen EB, Barratt-Due A, Haugaa H, Harboe M, Pischke SE, Nilsson PH, et al. The role of complement in liver injury, regeneration, and transplantation. Hepatology. 2019; 70:725–736. PMID: 30653682.4. Kaimori A, Potter J, Kaimori JY, Wang C, Mezey E, Koteish A. Transforming growth factor-beta1 induces an epithelial-to-mesenchymal transition state in mouse hepatocytes in vitro. J Biol Chem. 2007; 282:22089–22101. PMID: 17513865.5. Lu K, Liu G, Yang L, Liu F, Gao L, Shi J, et al. Sustainable inflammation transforms hepatic cells by causing oxidative stress injury and potential epithelial-mesenchymal transition. Int J Oncol. 2016; 49:971–980. PMID: 27315196.6. Xue ZF, Wu XM, Liu M. Hepatic regeneration and the epithelial to mesenchymal transition. World J Gastroenterol. 2013; 19:1380–1386. PMID: 23538893.7. Chen Z, Li S, Subramaniam S, Shyy JY, Chien S. Epigenetic regulation: a new frontier for biomedical engineers. Annu Rev Biomed Eng. 2017; 19:195–219. PMID: 28301736.8. Kelly TK, De Carvalho DD, Jones PA. Epigenetic modifications as therapeutic targets. Nat Biotechnol. 2010; 28:1069–1078. PMID: 20944599.9. Paluch BE, Naqash AR, Brumberger Z, Nemeth MJ, Griffiths EA. Epigenetics: a primer for clinicians. Blood Rev. 2016; 30:285–295. PMID: 26969414.10. Bedi U, Mishra VK, Wasilewski D, Scheel C, Johnsen SA. Epigenetic plasticity: a central regulator of epithelial-to-mesenchymal transition in cancer. Oncotarget. 2014; 5:2016–2029. PMID: 24840099.11. Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008; 14:818–829. PMID: 18539112.12. Lee CW, Chen YF, Wu HH, Lee OK. Historical perspectives and advances in mesenchymal stem cell research for the treatment of liver diseases. Gastroenterology. 2018; 154:46–56. PMID: 29107021.13. Dhawan A, Chaijitraruch N, Fitzpatrick E, Bansal S, Filippi C, Lehec SC, et al. Alginate microencapsulated human hepatocytes for the treatment of acute liver failure in children. J Hepatol. 2020; 72:877–884. PMID: 31843649.14. Ho CM, Chen YH, Chien CS, Ho SL, Chen HL, Hu RH, et al. Hepatocyte and mesenchymal stem cell co-transplantation in rats with acute liver failure. Korean J Transplant. 2020; 34:100–108. PMID: 35769351.15. Mu N, Liu HB, Meng QH, Du DW, Jiang Y, Hu HZ. The differentiation of human multipotent adult progenitor cells into hepatocyte-like cells induced by coculture with human hepatocyte line L02. Ann Surg Treat Res. 2015; 88:1–7. PMID: 25553318.16. Buisson EM, Park SH, Kim M, Kang K, Yoon S, Lee JE, et al. Transplantation of patient-specific bile duct bioengineered with chemically reprogrammed and microtopographically differentiated cells. Bioeng Transl Med. 2021; 7:e10252. PMID: 35079629.17. Kim Y, Hong SA, Yu J, Eom J, Jang K, Yoon S, et al. Adenine base editing and prime editing of chemically derived hepatic progenitors rescue genetic liver disease. Cell Stem Cell. 2021; 28:1614–1624. PMID: 33951479.18. Salas-Silva S, Kim Y, Kim TH, Kim M, Seo D, Choi J, et al. Human chemically-derived hepatic progenitors (hCdHs) as a source of liver organoid generation: application in regenerative medicine, disease modeling, and toxicology testing. Biomaterials. 2023; 303:122360. PMID: 38465578.19. Nie YZ, Zheng YW, Taniguchi H. Improving the repopulation capacity of elderly human hepatocytes by decoding aging-associated hepatocyte plasticity. Hepatology. 2022; 76:1030–1045. PMID: 35243665.20. Zhang W, Qu J, Liu GH, Belmonte JC. The ageing epigenome and its rejuvenation. Nat Rev Mol Cell Biol. 2020; 21:137–150. PMID: 32020082.21. Tanimizu N, Ichinohe N, Yamamoto M, Akiyama H, Nishikawa Y, Mitaka T. Progressive induction of hepatocyte progenitor cells in chronically injured liver. Sci Rep. 2017; 7:39990. PMID: 28051157.22. Antoniou A, Raynaud P, Cordi S, Zong Y, Tronche F, Stanger BZ, et al. Intrahepatic bile ducts develop according to a new mode of tubulogenesis regulated by the transcription factor SOX9. Gastroenterology. 2009; 136:2325–2333. PMID: 19403103.23. Kim M, Kim Y, Silva ES, Adisasmita M, Kim KS, Jung YK, et al. Enhancing generation efficiency of liver organoids in a collagen scaffold using human chemically derived hepatic progenitors. Ann Hepatobiliary Pancreat Surg. 2023; 27:342–349. PMID: 37661098.24. Falahi F, Sgro A, Blancafort P. Epigenome engineering in cancer: fairytale or a realistic path to the clinic? Front Oncol. 2015; 5:22. PMID: 25705610.25. Mann DA. Epigenetics in liver disease. Hepatology. 2014; 60:1418–1425. PMID: 24633972.26. Shay JW, Roninson IB. Hallmarks of senescence in carcinogenesis and cancer therapy. Oncogene. 2004; 23:2919–2933. PMID: 15077154.27. Liu C, Liu L, Chen X, Cheng J, Zhang H, Shen J, et al. Sox9 regulates self-renewal and tumorigenicity by promoting symmetrical cell division of cancer stem cells in hepatocellular carcinoma. Hepatology. 2016; 64:117–129. PMID: 26910875.28. Pastore N, Huynh T, Herz NJ, Calcagni’ A, Klisch TJ, Brunetti L, et al. TFEB regulates murine liver cell fate during development and regeneration. Nat Commun. 2020; 11:2461. PMID: 32424153.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- CORRIGENDUM: Epigenetic modulation inhibits epithelial-mesenchymal transition-driven fibrogenesis and enhances characteristics of chemically-derived hepatic progenitors
- The efficacy of exosomes from human chemically derived hepatic progenitors in liver damage alleviation: a preclinical experimental study
- Targeting epithelial-mesenchymal transition pathway in hepatocellular carcinoma
- Consideration of EphA2 in relation to epithelial-mesenchymal transition in uterine endometrial cancer
- The Role of Epithelial-mesenchymal Transition in the Gastroenterology