Ann Lab Med.
2024 May;44(3):235-244. 10.3343/alm.2023.0178.
Primary Hyperoxaluria Screening and Monitoring: Quantitative Measurement of Plasma Oxalate by Gas Chromatography-Mass Spectrometry With High Sensitivity
- Affiliations
-
- 1Department of Paediatric Laboratory Medicine, The Hospital for Sick Children, Toronto, Ontario, Canada
- 2Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada
- KMID: 2555694
- DOI: http://doi.org/10.3343/alm.2023.0178
Abstract
- Background
Plasma oxalate measurements can be used for the screening and therapeutic monitoring of primary hyperoxaluria. We developed a gas chromatography-mass spectrometry (GC-MS) assay for plasma oxalate measurements with high sensitivity and suitable testing volumes for pediatric populations.
Methods
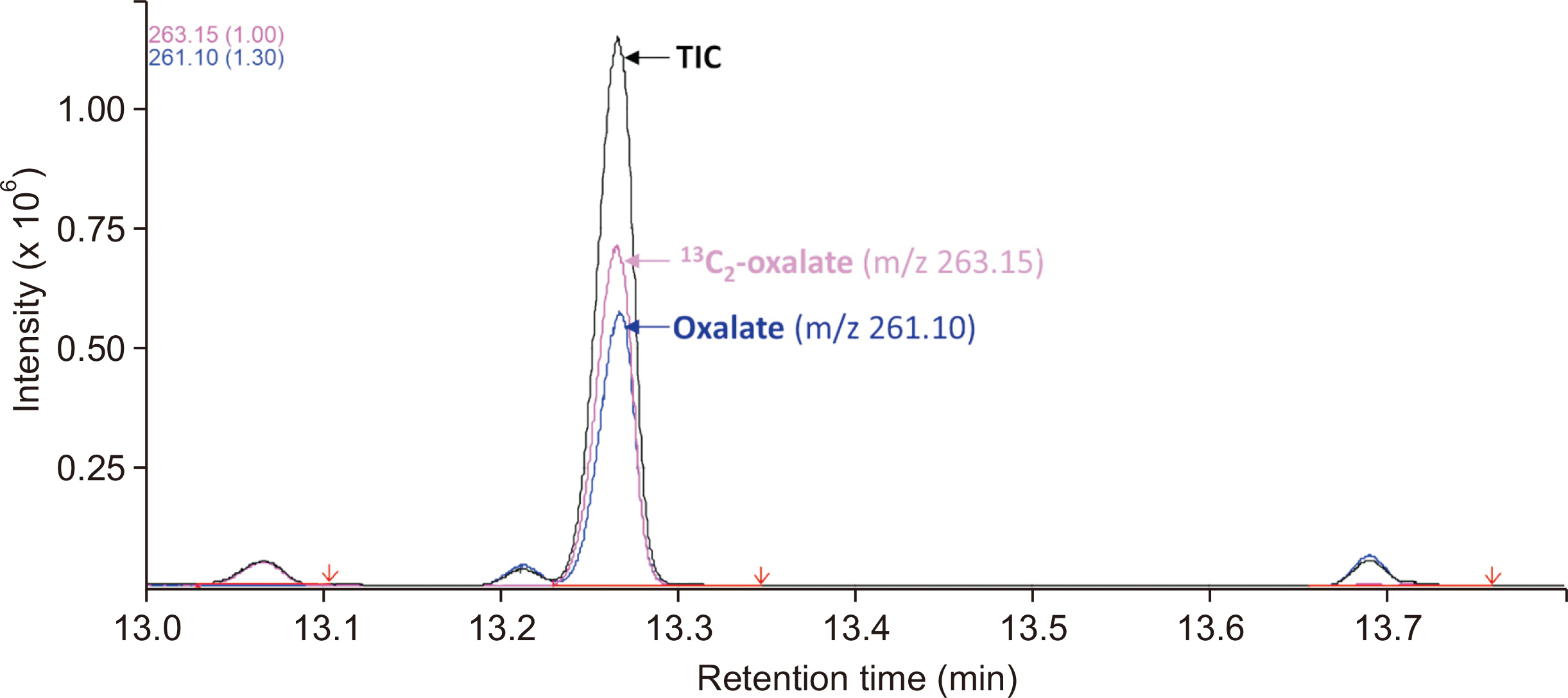
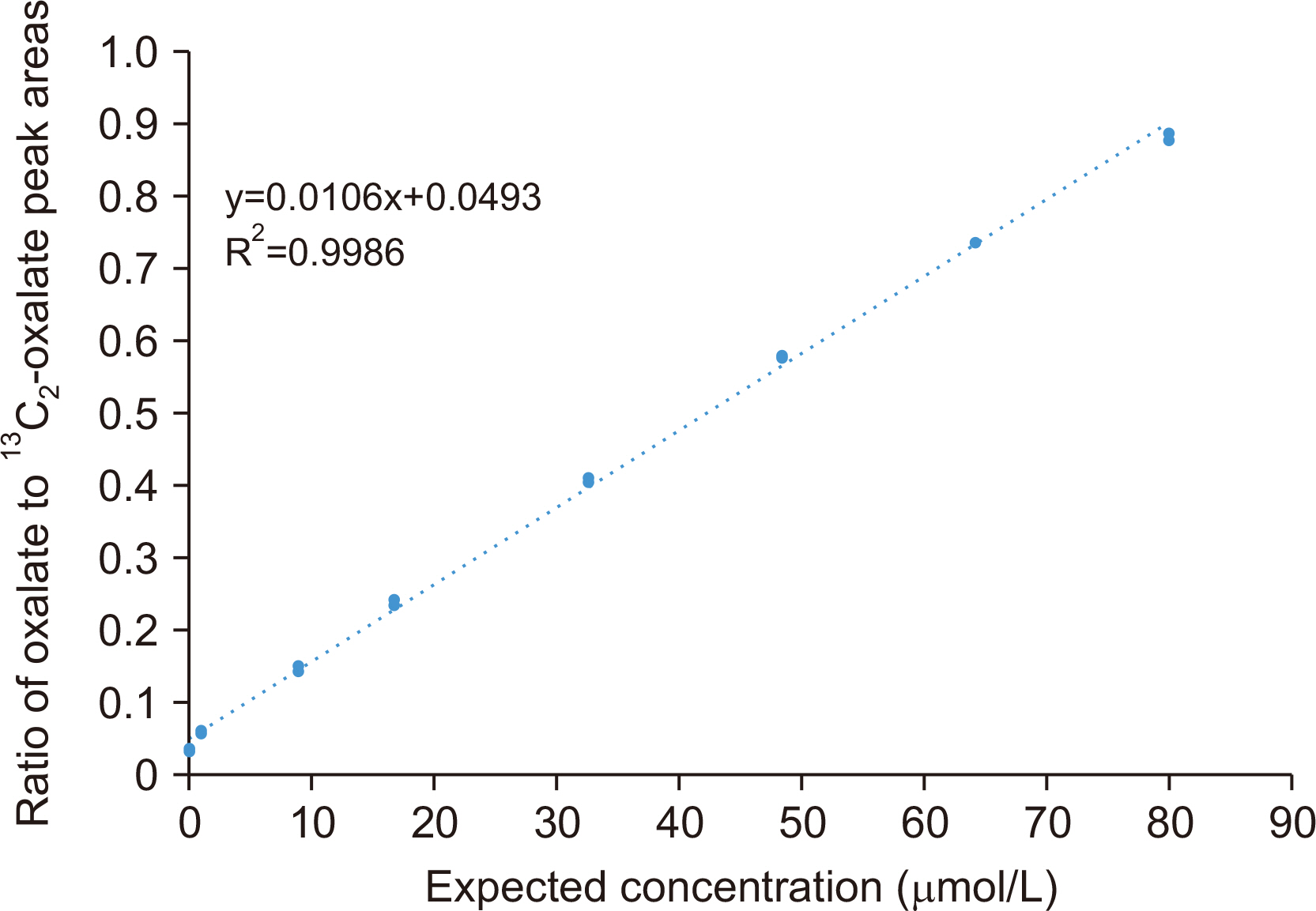
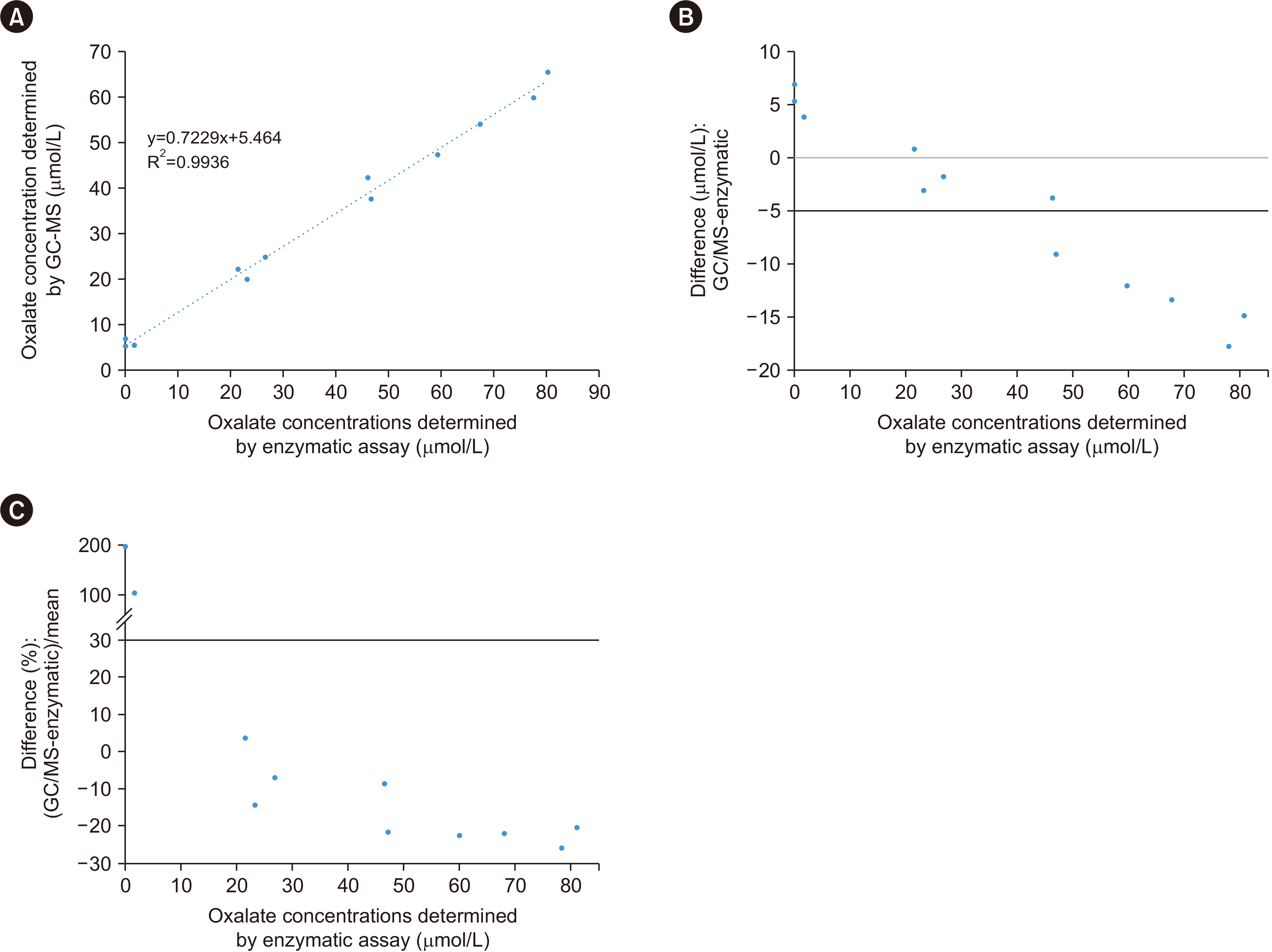
Plasma oxalate was extracted, derivatized, and analyzed by GC-MS. We measured the ion at m/z 261.10 to quantify oxalate and the 13 C 2-oxalate ion (m/z: 263.15) as the internal standard. Method validation included determination of the linear range, limit of blank, limit of detection, lower limit of quantification, precision, recovery, carryover, interference, and dilution effect. The cut-off value between primary and non-primary hyperoxaluria in a pediatric population was analyzed.
Results
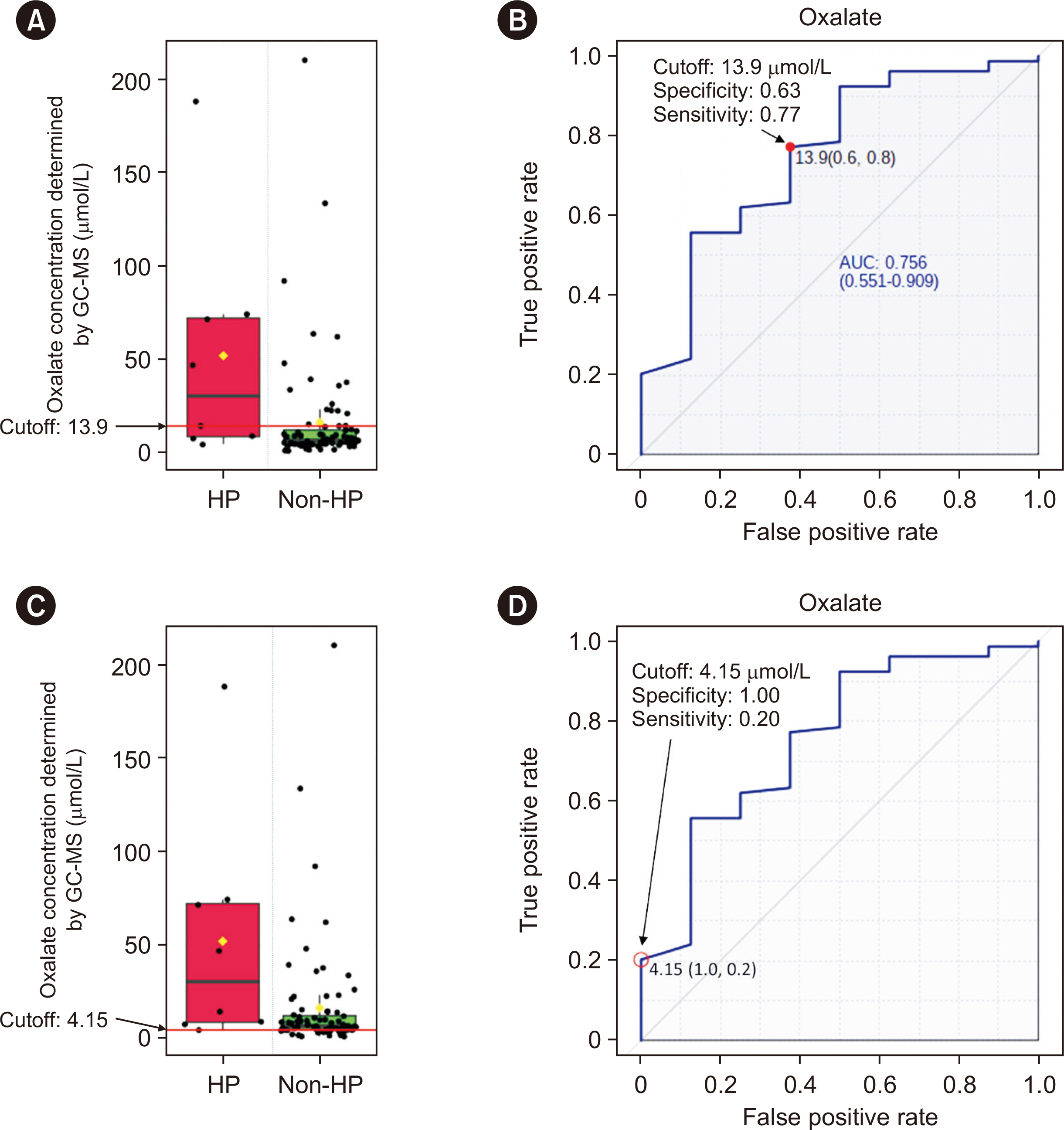
The detection limit was 0.78 μmol/L, and the linear range was up to 80.0 μmol/L. The between-day precision was 5.7% at 41.3 μmol/L and 13.1% at 1.6 μmol/L. The carry-over was < 0.2%. The recovery rate ranged from 90% to 110%. Interference analysis showed that Hb did not interfere with plasma oxalate quantification, whereas intralipids and bilirubin caused false elevation of oxalate concentrations. A cut-off of 13.9 μmol/L showed 63% specificity and 77% sensitivity, whereas a cut-off of 4.15 μmol/L showed 100% specificity and 20% sensitivity. The minimum required sample volume was 250 μL. The detected oxalate concentrations showed interference from instrument conditioning, sample preparation procedures, medications, and various clinical conditions.
Conclusions
GC-MS is a sensitive assay for quantifying plasma oxalate and is suitable for pediatric patients. Plasma oxalate concentrations should be interpreted in a clinical context.
Keyword
Figure
Reference
-
References
1. Brzica H, Breljak D, Burckhardt BC, Burckhardt G, Sabolić I. 2013; Oxalate: from the environment to kidney stones. Arh Hig Rada Toksikol. 64:609–30. DOI: 10.2478/10004-1254-64-2013-2428. PMID: 24384768.2. Danpure CJ, Cooper PJ, Wise PJ, Jennings PR. 1989; An enzyme trafficking defect in two patients with primary hyperoxaluria type 1: peroxisomal alanine/glyoxylate aminotransferase rerouted to mitochondria. J Cell Biol. 108:1345–52. DOI: 10.1083/jcb.108.4.1345. PMID: 2925788. PMCID: PMC2115519.3. Williams EL, Acquaviva C, Amoroso A, Chevalier F, Coulter-Mackie M, Monico CG, et al. 2009; Primary hyperoxaluria type 1: update and additional mutation analysis of the AGXT gene. Hum Mutat. 30:910–7. DOI: 10.1002/humu.21021. PMID: 19479957.4. Cochat P, Hulton SA, Acquaviva C, Danpure CJ, Daudon M, De Marchi M, et al. 2012; Primary hyperoxaluria type 1: indications for screening and guidance for diagnosis and treatment. Nephrol Dial Transplant. 27:1729–36. DOI: 10.1093/ndt/gfs078. PMID: 22547750.5. Cramer SD, Ferree PM, Lin K, Milliner DS, Holmes RP. 1999; The gene encoding hydroxypyruvate reductase (GRHPR) is mutated in patients with primary hyperoxaluria type II. Hum Mol Genet. 8:2063–9. DOI: 10.1093/hmg/8.11.2063. PMID: 10484776.6. Rumsby G, Cochat P. 2013; Primary hyperoxaluria. N Engl J Med. 369:2163. DOI: 10.1056/NEJMc1311606.7. Williams EL, Bockenhauer D, van't Hoff WG, Johri N, Laing C, Sinha MD, et al. 2012; The enzyme 4-hydroxy-2-oxoglutarate aldolase is deficient in primary hyperoxaluria type 3. Nephrol Dial Transplant. 27:3191–5. DOI: 10.1093/ndt/gfs039. PMID: 22391140.8. Greed L, Willis F, Johnstone L, Teo S, Belostotsky R, Frishberg Y, et al. 2018; Metabolite diagnosis of primary hyperoxaluria type 3. Pediatr Nephrol. 33:1443–6. DOI: 10.1007/s00467-018-3967-6. PMID: 29705963.9. Porowski T, Gałasiński W. 2003; A semi-micromethod for determination of oxalate in human plasma. Acta Pol Pharm. 60:239–45.10. Hoppe B, Kemper MJ, Hvizd MG, Sailer DE, Langman CB. 1998; Simultaneous determination of oxalate, citrate and sulfate in children's plasma with ion chromatography. Kidney Int. 53:1348–52. DOI: 10.1046/j.1523-1755.1998.00891.x. PMID: 9573551.11. Elgstoen KBP. 2008; Liquid chromatography-tandem mass spectrometry method for routine measurement of oxalic acid in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 873:31–6. DOI: 10.1016/j.jchromb.2008.07.002. PMID: 18755640.12. Chambers MM, Russell JC. 1973; A specific assay for plasma oxalate. Clin Biochem. 6:22–8. DOI: 10.1016/S0009-9120(73)80005-0. PMID: 4699617.13. France NC, Holland PT, McGhie TK, Wallace MR. 1988; Measurement of plasma oxalate by capillary gas chromatography and its validation by isotope dilution mass spectrometry. J Chromatogr. 433:1–7. DOI: 10.1016/S0378-4347(00)80579-4. PMID: 3069854.14. Inoue Y, Masuyama H, Ikawa H, Mitsubuchi H, Kuhara T. 2003; Monitoring method for pre- and post-liver transplantation in patients with primary hyperoxaluria type I. J Chromatogr B Analyt Technol Biomed Life Sci. 792:89–97. DOI: 10.1016/S1570-0232(03)00278-2. PMID: 12829001.15. Wolthers BG, Hayer M. 1982; The determination of oxalic acid in plasma and urine by means of capillary gas chromatography. Clin Chim Acta. 120:87–102. DOI: 10.1016/0009-8981(82)90080-8. PMID: 7067141.16. Ladwig PM, Liedtke RR, Larson TS, Lieske JC. 2005; Sensitive spectrophotometric assay for plasma oxalate. Clin Chem. 51:2377–80. DOI: 10.1373/clinchem.2005.054353. PMID: 16306102.17. Xia J, Psychogios N, Young N, Wishart DS. 2009; MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 37(Web Server issue):W652–60. DOI: 10.1093/nar/gkp356. PMID: 19429898. PMCID: PMC2703878.18. De Nicolò A, Cantù M, D'Avolio A. 2017; Matrix effect management in liquid chromatography mass spectrometry: the internal standard normalized matrix effect. Bioanalysis. 9:1093–105. DOI: 10.4155/bio-2017-0059. PMID: 28737421.19. Cortese M, Gigliobianco MR, Magnoni F, Censi R, Di Martino PD. 2020; Compensate for or minimize matrix effects? Strategies for overcoming matrix effects in liquid chromatography-mass spectrometry technique: a tutorial review. Molecules. 25:3047. DOI: 10.3390/molecules25133047. PMID: 32635301. PMCID: PMC7412464.20. Stokes F, Acquaviva-Bourdain C, Hoppe B, Lieske JC, Lindner E, Toulson G, et al. 2020; Plasma oxalate: comparison of methodologies. Urolithiasis. 48:473–80. DOI: 10.1007/s00240-020-01197-4. PMID: 32472220. PMCID: PMC7666277.21. Assimos DG. 2020; Re: plasma oxalate: comparison of methodologies. J Urol. 204:1374. DOI: 10.1097/JU.0000000000001280.22. Mazzachi BC, Teubner JK, Ryall RL. 1984; Factors affecting measurement of urinary oxalate. Clin Chem. 30:1339–43. DOI: 10.1093/clinchem/30.8.1339. PMID: 6744582.23. Hellman L, Burns JJ. 1958; Metabolism of l-ascorbic acid-1-C14 in man. J Biol Chem. 230:923–30. DOI: 10.1016/S0021-9258(18)70515-2. PMID: 13525409.24. Knight J, Madduma-Liyanage K, Mobley JA, Assimos DG, Holmes RP. 2016; Ascorbic acid intake and oxalate synthesis. Urolithiasis. 44:289–97. DOI: 10.1007/s00240-016-0868-7. PMID: 27002809. PMCID: PMC4946963.25. Rolton HA, McConnell KM, Modi KS, Macdougall AI. 1991; The effect of vitamin C intake on plasma oxalate in patients on regular haemodialysis. Nephrol Dial Transplant. 6:440–3. DOI: 10.1093/ndt/6.6.440. PMID: 1876286.26. Wechtersbach L, Cigić B. 2007; Reduction of dehydroascorbic acid at low pH. J Biochem Biophys Methods. 70:767–72. DOI: 10.1016/j.jbbm.2007.04.007. PMID: 17544513.27. Simpson GL, Ortwerth BJ. 2000; The non-oxidative degradation of ascorbic acid at physiological conditions. Biochim Biophys Acta. 1501:12–24. DOI: 10.1016/S0925-4439(00)00009-0. PMID: 10727845.28. Kasidas GP, Rose GA. 1986; Measurement of plasma oxalate in healthy subjects and in patients with chronic renal failure using immobilised oxalate oxidase. Clin Chim Acta. 154:49–58. DOI: 10.1016/0009-8981(86)90087-2. PMID: 3943224.29. Perinpam M, Enders FT, Mara KC, Vaughan LE, Mehta RA, Voskoboev N, et al. 2017; Plasma oxalate in relation to eGFR in patients with primary hyperoxaluria, enteric hyperoxaluria and urinary stone disease. Clin Biochem. 50:1014–9. DOI: 10.1016/j.clinbiochem.2017.07.017. PMID: 28764885. PMCID: PMC5705406.30. Costello JF, Sadovnic MJ, Cottington EM. 1991; Plasma oxalate concentrations rise in hemodialysis patients despite increased oxalate removal. J Am Soc Nephrol. 1:1289–98. DOI: 10.1681/ASN.V1121289. PMID: 1912391.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A screening method for neuroblastoma and organic acidemias by gas chromatography-mass spectrometry
- Quantitative analysis of endogenous steroids in human urine by using gas chromatography-mass spectrometry
- A Case of Primary Hyperoxaluria with Renal Allograft Dysfunction
- Quantitative Analysis of Urinary Organic Acids by Gas Chromatography-Mass Spectrometry
- End-stage Renal Disease Caused by Primary Hyperoxaluria