Korean J Physiol Pharmacol.
2024 May;28(3):253-264. 10.4196/kjpp.2024.28.3.253.
Relation between heart rate variability and spectral analysis of electroencephalogram in chronic neuropathic pain patients
- Affiliations
-
- 1Department of Physiology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry 605006, India
- 2Department of Anesthesiology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry 605006, India
- KMID: 2555667
- DOI: http://doi.org/10.4196/kjpp.2024.28.3.253
Abstract
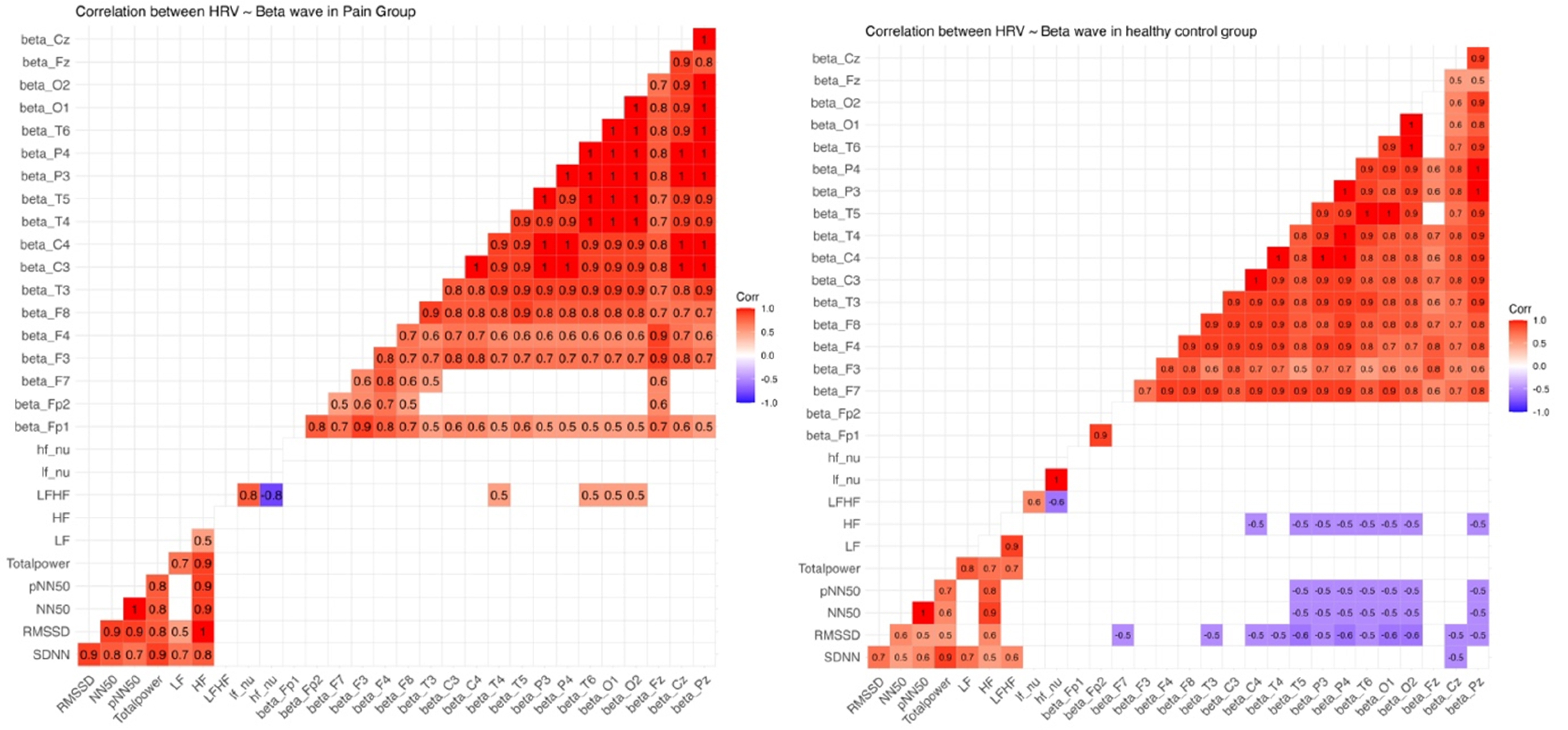
- Chronic neuropathic pain (CNP) is a complex condition often arising from neural maladaptation after nerve injury. Understanding CNP complications involves the intricate interplay between brain-heart dynamics, assessed through quantitative electroencephalogram (qEEG) and heart rate variability (HRV). However, insights into their interaction in chronic pain are limited. Resting EEG and simultaneous electrocardiogram (lead II) of the participants were recorded for qEEG and HRV analysis. Correlations between HRV and qEEG parameters were calculated and compared with age, sex, and body mass index (BMI)-matched controls. CNP patients showed reduced HRV and significant increases in qEEG power spectral densities within delta, theta, and beta frequency ranges. A positive correlation was found between low frequency/high frequency (LF/HF) ratio in HRV analysis and theta, alpha, and beta frequency bands in qEEG among CNP patients. However, no significant correlation was observed between parasympathetic indices and theta, beta bands in qEEG within CNP group, unlike age, sex, and BMI-matched healthy controls. CNP patients display significant HRV reductions and distinctive qEEG patterns. While healthy controls exhibit significant correlations between parasympathetic HRV parameters and qEEG spectral densities, these relationships are diminished or absent in CNP individuals. LF/HF ratio, reflecting sympathovagal balance, correlates significantly with qEEG frequency bands (theta, alpha, beta), illuminating autonomic dysregulation in CNP. These findings emphasize the intricate brain-heart interplay in chronic pain, warranting further exploration.
Keyword
Figure
Reference
-
1. Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, Freeman R, Truini A, Attal N, Finnerup NB, Eccleston C, Kalso E, Bennett DL, Dworkin RH, Raja SN. 2017; Neuropathic pain. Nat Rev Dis Primers. 3:17002. DOI: 10.1038/nrdp.2017.2. PMID: 28205574. PMCID: PMC5371025.
Article2. van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. 2014; Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 155:654–662. DOI: 10.1016/j.pain.2013.11.013. PMID: 24291734.
Article3. de Moraes Vieira EB, Garcia JB, da Silva AA, Mualem Araújo RL, Jansen RC. 2012; Prevalence, characteristics, and factors associated with chronic pain with and without neuropathic characteristics in São Luís, Brazil. J Pain Symptom Manage. 44:239–251. DOI: 10.1016/j.jpainsymman.2011.08.014. PMID: 22871508.
Article4. Dieleman JP, Kerklaan J, Huygen FJPM, Bouma PAD, Sturkenboom MCJM. 2008; Incidence rates and treatment of neuropathic pain conditions in the general population. Pain. 137:681–688. DOI: 10.1016/j.pain.2008.03.002. PMID: 18439759.
Article5. Fine PG. 2011; Long-term consequences of chronic pain: mounting evidence for pain as a neurological disease and parallels with other chronic disease states. Pain Med. 12:996–1004. DOI: 10.1111/j.1526-4637.2011.01187.x. PMID: 21752179.
Article6. Karri J, Li S, Chen YT, Stampas A, Li S. 2021; Observations of autonomic variability following central neuromodulation for chronic neuropathic pain in spinal cord injury. Neuromodulation. 24:427–433. DOI: 10.1111/ner.12979. PMID: 31199549. PMCID: PMC6911028.
Article7. Jensen MP, Sherlin LH, Gertz KJ, Braden AL, Kupper AE, Gianas A, Howe JD, Hakimian S. 2013; Brain EEG activity correlates of chronic pain in persons with spinal cord injury: clinical implications. Spinal Cord. 51:55–58. DOI: 10.1038/sc.2012.84. PMID: 22801188.
Article8. Tayeb Z, Bose R, Dragomir A, Osborn LE, Thakor NV, Cheng G. 2020; Decoding of pain perception using EEG signals for a real-time reflex system in prostheses: a case study. Sci Rep. 10:5606. DOI: 10.1038/s41598-020-62525-7. PMID: 32221336. PMCID: PMC7101312.
Article9. Pinheiro ES, de Queirós FC, Montoya P, Santos CL, do Nascimento MA, Ito CH, Silva M, Nunes Santos DB, Benevides S, Miranda JG, Sá KN, Baptista AF. 2016; Electroencephalographic patterns in chronic pain: a systematic review of the literature. PLoS One. 11:e0149085. DOI: 10.1371/journal.pone.0149085. PMID: 26914356. PMCID: PMC4767709.
Article10. Forte G, Troisi G, Pazzaglia M, Pascalis V, Casagrande M. 2022; Heart rate variability and pain: a systematic review. Brain Sci. 12:153. DOI: 10.3390/brainsci12020153. PMID: 35203917. PMCID: PMC8870705.
Article11. Yetwin A, Marks K, Bell T, Gold J. 2012; Heart rate variability biofeedback therapy for children and adolescents with chronic pain. J Pain. 13:S93. DOI: 10.1016/j.jpain.2012.01.386.
Article12. Södervall J, Karppinen J, Puolitaival J, Kyllönen E, Kiviniemi AM, Tulppo MP, Hautala AJ. 2013; Heart rate variability in sciatica patients referred to spine surgery: a case control study. BMC Musculoskelet Disord. 14:149. DOI: 10.1186/1471-2474-14-149. PMID: 23622100. PMCID: PMC3644230.
Article13. Karri J, Zhang L, Li S, Chen YT, Stampas A, Li S. 2017; Heart rate variability: a novel modality for diagnosing neuropathic pain after spinal cord injury. Front Physiol. 8:495. DOI: 10.3389/fphys.2017.00495. PMID: 28769815. PMCID: PMC5513934.
Article14. Dworkin RH, O'Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, Kalso EA, Loeser JD, Miaskowski C, Nurmikko TJ, Portenoy RK, Rice ASC, Stacey BR, Treede RD, Turk DC, Wallace MS. 2007; Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 132:237–251. DOI: 10.1016/j.pain.2007.08.033. PMID: 17920770.
Article15. Bushnell MC, Frangos E, Madian N. 2021; Non-pharmacological treatment of pain: grand challenge and future opportunities. Front Pain Res (Lausanne). 2:696783. DOI: 10.3389/fpain.2021.696783. PMID: 35295445. PMCID: PMC8915661.
Article16. El Geziry A, Toble Y, Al Kadhi F, Pervaiz M, Al Nobani M. Shallik NA, editor. 2018. Non-pharmacological pain management. Pain management in special circumstances. IntechOpen;DOI: 10.5772/intechopen.79689.
Article17. Hulley SB, Cummings SR, Browner WS, Grady DG, Newman TB. 2013. Designing clinical research. 4th ed. Wolters Kluwer/Lippincott Williams & Wilkins.18. Kane N, Acharya J, Benickzy S, Caboclo L, Finnigan S, Kaplan PW, Shibasaki H, Pressler R, van Putten MJAM. 2017; A revised glossary of terms most commonly used by clinical electroencephalographers and updated proposal for the report format of the EEG findings. Revision 2017. Clin Neurophysiol Pract. 2:170–185. Erratum in: Clin Neurophysiol Pract. 2019;4:133. DOI: 10.1016/j.cnp.2019.06.001. PMID: 31309168. PMCID: PMC6606822.
Article19. 1994; Guideline fourteen: guidelines for recording clinical EEG on digital media. American Electroencephalographic Society. J Clin Neurophysiol. 11:114–115. DOI: 10.1097/00004691-199401000-00015.20. 1974; A glossary of terms most commonly used by clinical electroencephalographers. Electroencephalogr Clin Neurophysiol. 37:538–548. DOI: 10.1016/0013-4694(74)90099-6. PMID: 4138729.21. Kang JH, Kim JK, Hong SH, Lee CH, Choi BY. 2016; Heart rate variability for quantification of autonomic dysfunction in fibromyalgia. Ann Rehabil Med. 40:301–309. DOI: 10.5535/arm.2016.40.2.301. PMID: 27152281. PMCID: PMC4855125.
Article22. Hazra S, Venkataraman S, Handa G, Yadav SL, Wadhwa S, Singh U, Kochhar KP, Deepak KK, Sarkar K. 2020; A cross-sectional study on central sensitization and autonomic changes in fibromyalgia. Front Neurosci. 14:788. DOI: 10.3389/fnins.2020.00788. PMID: 32848561. PMCID: PMC7417433.
Article23. Singh SK, Roy A. 2017; Assessment of heart rate variability in the patients suffering with chronic pain of musculoskeletal origin. Natl J Physiol Pharm Pharmacol. 7:712–712. DOI: 10.5455/njppp.2017.7.0204803032017.
Article24. Chinthakanan S, Laosuwan K, Boonyawong P, Kumfu S, Chattipakorn N, Chattipakorn SC. 2018; Reduced heart rate variability and increased saliva cortisol in patients with TMD. Arch Oral Biol. 90:125–129. DOI: 10.1016/j.archoralbio.2018.03.011. PMID: 29604544.
Article25. Eze-Nliam CM, Quartana PJ, Quain AM, Smith MT. 2011; Nocturnal heart rate variability is lower in temporomandibular disorder patients than in healthy, pain-free individuals. J Orofac Pain. 25:232–239.26. Hallman DM, Lindberg LG, Arnetz BB, Lyskov E. 2011; Effects of static contraction and cold stimulation on cardiovascular autonomic indices, trapezius blood flow and muscle activity in chronic neck-shoulder pain. Eur J Appl Physiol. 111:1725–1735. Erratum in: Eur J Appl Physiol. 2011;111:1737. DOI: 10.1007/s00421-010-1813-z. PMID: 21221987.
Article27. Kulshreshtha P, Gupta R, Yadav RK, Bijlani RL, Deepak KK. 2012; A comprehensive study of autonomic dysfunction in the fibromyalgia patients. Clin Auton Res. 22:117–122. DOI: 10.1007/s10286-011-0150-6. PMID: 22038566.
Article28. Lerma C, Martinez A, Ruiz N, Vargas A, Infante O, Martinez-Lavin M. 2011; Nocturnal heart rate variability parameters as potential fibromyalgia biomarker: correlation with symptoms severity. Arthritis Res Ther. 13:R185. DOI: 10.1186/ar3513. PMID: 22087605. PMCID: PMC3334634.
Article29. Zamunér AR, Forti M, Andrade CP, Avila MA, da Silva E. 2016; Respiratory sinus arrhythmia and its association with pain in women with fibromyalgia syndrome. Pain Pract. 16:704–711. DOI: 10.1111/papr.12321. PMID: 26032241.
Article30. Koenig J, Jarczok MN, Ellis RJ, Hillecke TK, Thayer JF. 2014; Heart rate variability and experimentally induced pain in healthy adults: a systematic review. Eur J Pain. 18:301–314. DOI: 10.1002/j.1532-2149.2013.00379.x. PMID: 23922336.
Article31. Koenig J, Loerbroks A, Jarczok MN, Fischer JE, Thayer JF. 2016; Chronic pain and heart rate variability in a cross-sectional occupational sample: evidence for impaired vagal control. Clin J Pain. 32:218–225. DOI: 10.1097/AJP.0000000000000242. PMID: 25924095.
Article32. Rampazo ÉP, Rehder-Santos P, Catai AM, Liebano RE. 2024; Heart rate variability in adults with chronic musculoskeletal pain: a systematic review. Pain Pract. 24:211–230. DOI: 10.1111/papr.13294. PMID: 37661339.
Article33. Malik M, Bigger JT, Camm AJ, Kleiger RE, Malliani A, Moss AJ, Schwartz PJ. 1996; Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 17:354–381. DOI: 10.1093/oxfordjournals.eurheartj.a014868.
Article34. Burr RL. 2007; Interpretation of normalized spectral heart rate variability indices in sleep research: a critical review. Sleep. 30:913–919. DOI: 10.1093/sleep/30.7.913. PMID: 17682663. PMCID: PMC1978375.
Article35. Abhang PA, Gawali BW, Mehrotra SC. Abhang PA, Gawali BW, Mehrotra SC, editors. 2016. Chapter 3 - technical aspects of brain rhythms and speech parameters. Introduction to EEG- and speech-based emotion recognition. Academic Press;p. 51–79. DOI: 10.1016/B978-0-12-804490-2.00003-8.36. Drewes AM, Nielsen KD, Arendt-Nielsen L, Birket-Smith L, Hansen LM. 1997; The effect of cutaneous and deep pain on the electroencephalogram during sleep--an experimental study. Sleep. 20:632–640. DOI: 10.1093/sleep/20.8.632. PMID: 9351131.
Article37. Meerwijk EL, Ford JM, Weiss SJ. 2015; Resting-state EEG delta power is associated with psychological pain in adults with a history of depression. Biol Psychol. 105:106–114. DOI: 10.1016/j.biopsycho.2015.01.003. PMID: 25600291. PMCID: PMC4336814.
Article38. Mussigmann T, Bardel B, Lefaucheur JP. 2022; Resting-state electroencephalography (EEG) biomarkers of chronic neuropathic pain. A systematic review. Neuroimage. 258:119351. DOI: 10.1016/j.neuroimage.2022.119351. PMID: 35659993.
Article39. Sarnthein J, Stern J, Aufenberg C, Rousson V, Jeanmonod D. 2006; Increased EEG power and slowed dominant frequency in patients with neurogenic pain. Brain. 129:55–64. DOI: 10.1093/brain/awh631. PMID: 16183660.
Article40. Boord P, Siddall PJ, Tran Y, Herbert D, Middleton J, Craig A. 2008; Electroencephalographic slowing and reduced reactivity in neuropathic pain following spinal cord injury. Spinal Cord. 46:118–123. DOI: 10.1038/sj.sc.3102077. PMID: 17502876.
Article41. Stern J, Jeanmonod D, Sarnthein J. 2006; Persistent EEG overactivation in the cortical pain matrix of neurogenic pain patients. Neuroimage. 31:721–731. DOI: 10.1016/j.neuroimage.2005.12.042. PMID: 16527493.
Article42. Michels L, Moazami-Goudarzi M, Jeanmonod D. 2011; Correlations between EEG and clinical outcome in chronic neuropathic pain: surgical effects and treatment resistance. Brain Imaging Behav. 5:329–348. DOI: 10.1007/s11682-011-9135-2. PMID: 21948245.
Article43. Vuckovic A, Jajrees M, Purcell M, Berry H, Fraser M. 2018; Electroencephalographic predictors of neuropathic pain in subacute spinal cord injury. J Pain. 19:1256.e1–1256.e17. DOI: 10.1016/j.jpain.2018.04.011. PMID: 29751110.
Article44. Di Pietro F, Macey PM, Rae CD, Alshelh Z, Macefield VG, Vickers ER, Henderson LA. 2018; The relationship between thalamic GABA content and resting cortical rhythm in neuropathic pain. Hum Brain Mapp. 39:1945–1956. DOI: 10.1002/hbm.23973. PMID: 29341331. PMCID: PMC6866606.
Article45. Krupina NA, Churyukanov MV, Kukushkin ML, Yakhno NN. 2020; Central neuropathic pain and profiles of quantitative electroencephalography in multiple sclerosis patients. Front Neurol. 10:1380. DOI: 10.3389/fneur.2019.01380. PMID: 32038459. PMCID: PMC6990108.
Article46. van den Broeke EN, Wilder-Smith OH, van Goor H, Vissers KC, van Rijn CM. 2013; Patients with persistent pain after breast cancer treatment show enhanced alpha activity in spontaneous EEG. Pain Med. 14:1893–1899. DOI: 10.1111/pme.12216. PMID: 24034712.
Article47. Kisler LB, Kim JA, Hemington KS, Rogachov A, Cheng JC, Bosma RL, Osborne NR, Dunkley BT, Inman RD, Davis KD. 2020; Abnormal alpha band power in the dynamic pain connectome is a marker of chronic pain with a neuropathic component. Neuroimage Clin. 26:102241. DOI: 10.1016/j.nicl.2020.102241. PMID: 32203904. PMCID: PMC7090370.
Article48. Simis M, Imamura M, Pacheco-Barrios K, Marduy A, de Melo PS, Mendes AJ, Teixeira PEP, Battistella L, Fregni F. 2022; EEG theta and beta bands as brain oscillations for different knee osteoarthritis phenotypes according to disease severity. Sci Rep. 12:1480. DOI: 10.1038/s41598-022-04957-x. PMID: 35087082. PMCID: PMC8795380.
Article49. Zebhauser PT, Hohn VD, Ploner M. 2023; Resting-state electroencephalography and magnetoencephalography as biomarkers of chronic pain: a systematic review. Pain. 164:1200–1221. DOI: 10.1097/j.pain.0000000000002825. PMID: 36409624. PMCID: PMC10184564.
Article50. Jeanmonod D, Schulman J, Ramirez R, Cancro R, Lanz M, Morel A, Magnin M, Siegemund M, Kronberg E, Ribary U, Llinas R. 2003; Neuropsychiatric thalamocortical dysrhythmia: surgical implications. Neurosurg Clin N Am. 14:251–265. DOI: 10.1016/S1042-3680(02)00116-X. PMID: 12856492.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Determinants of Heart Rate Variability in General Korean Population
- 24 Hours Heart Rate Variability in Elderly Hypertensive Patients
- Validity of Heart Rate Variability Using Poincare Plot for Assessing Vagal Tone during General Anesthesia
- Characteristics of electroencephalogram signatures in sedated patients induced by various anesthetic agents
- Importance and challenges of neuropathic pain