Korean J Physiol Pharmacol.
2024 May;28(3):219-227. 10.4196/kjpp.2024.28.3.219.
Novel artesunate-metformin conjugate inhibits bladder cancer cell growth associated with Clusterin/SREBP1/FASN signaling pathway
- Affiliations
-
- 1Key Laboratory of Study and Discovery of Small Targeted Molecules of Hunan Province, Department of Pharmacy, School of Medicine, Hunan Normal University, Changsha 410000, Hunan, China
- KMID: 2555664
- DOI: http://doi.org/10.4196/kjpp.2024.28.3.219
Abstract
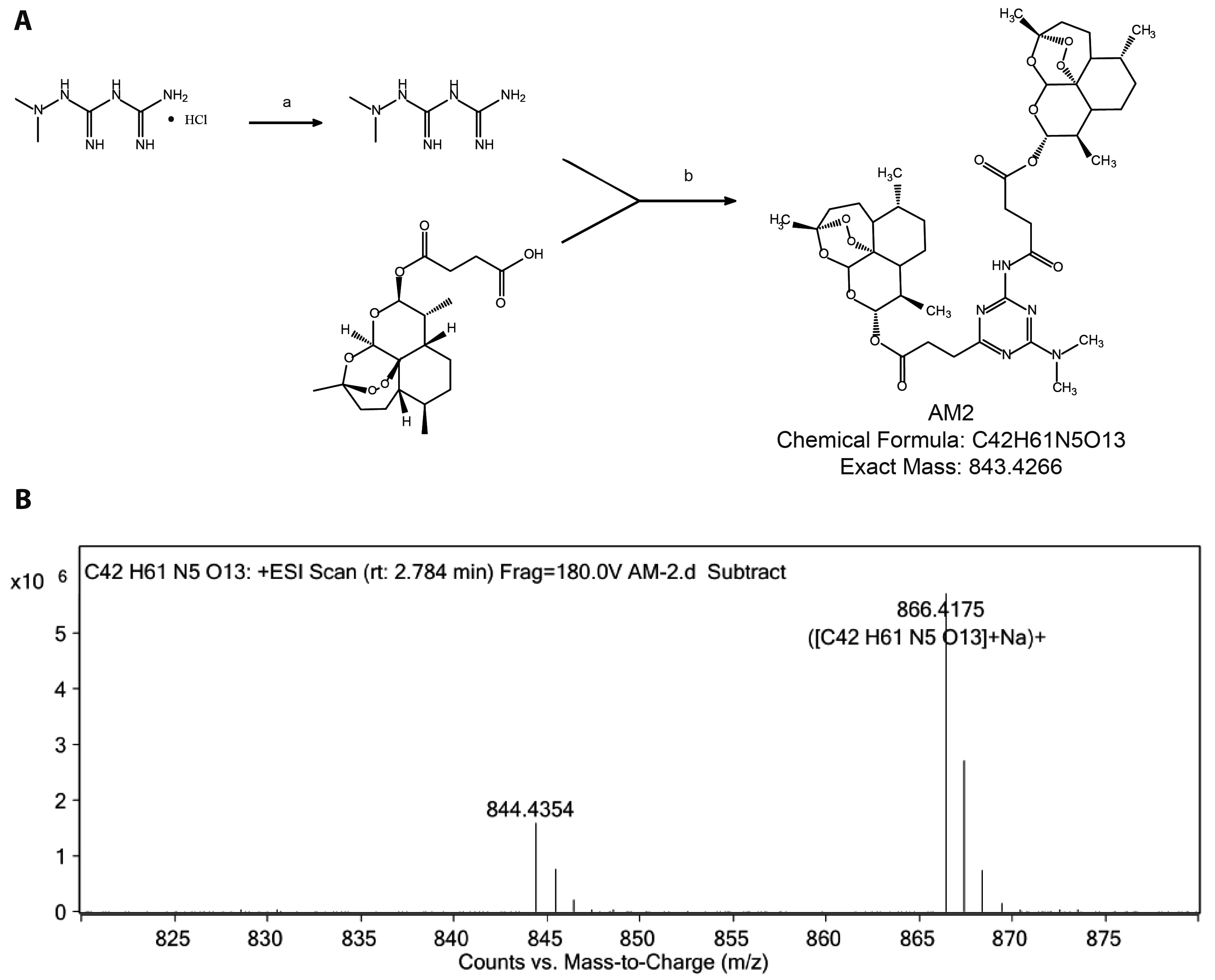
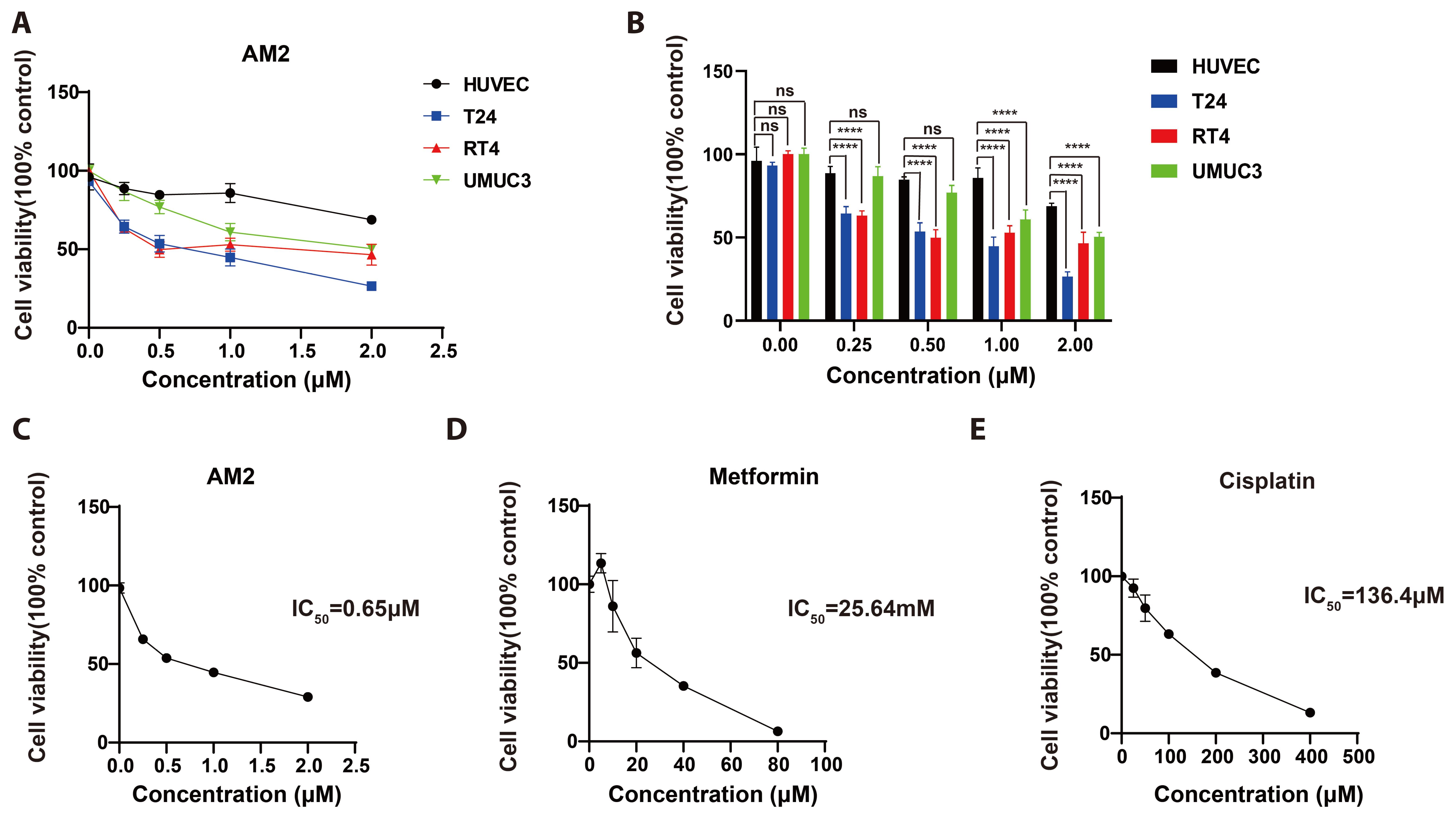
- Bladder cancer remains the 10th most common cancer worldwide. In recent years, metformin has been found to have potential anti-bladder cancer activ- ity while high concentration of IC50 at millimolar level is needed, which could not be reached by regular oral administration route. Thus, higher efficient agent is urgently demanded for clinically treating bladder cancer. Here, by conjugating artesunate to metformin, a novel artesunate-metformin dimer triazine derivative AM2 was designed and synthesized. The inhibitory effect of AM2 on bladder cancer cell line T24 and the mechanism underlying was determined. Anti-tumor activity of AM2 was assessed by MTT, cloning formation and wound healing assays. Decreasing effect of AM2 on lipogenesis was determined by oil red O staining. The protein expressions of Clusterin, SREBP1 and FASN in T24 cells were evaluated by Western blotting. The results show that AM2 significantly inhibited cell proliferation and migration at micromolar level, much higher than parental metformin. AM2 reduced lipogenesis and down-regulated the expressions of Clusterin, SREBP1 and FASN. These results suggest that AM2 inhibits the growth of bladder cancer cells T24 by inhibiting cellular lipogenesis associated with the Clusterin/SREBP1/FASN signaling pathway.
Keyword
Figure
Reference
-
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. 2021; Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71:209–249. DOI: 10.3322/caac.21660. PMID: 33538338.
Article2. Tran L, Xiao JF, Agarwal N, Duex JE, Theodorescu D. 2021; Advances in bladder cancer biology and therapy. Nat Rev Cancer. 21:104–121. DOI: 10.1038/s41568-020-00313-1. PMID: 33268841. PMCID: PMC10112195.
Article3. Yin M, Joshi M, Meijer RP, Glantz M, Holder S, Harvey HA, Kaag M, Fransen van de Putte EE, Horenblas S, Drabick JJ. 2016; Neoadjuvant chemotherapy for muscle-invasive bladder cancer: a systematic review and two-step meta-analysis. Oncologist. 21:708–715. DOI: 10.1634/theoncologist.2015-0440. PMID: 27053504. PMCID: PMC4912364.
Article4. Crona DJ, Faso A, Nishijima TF, McGraw KA, Galsky MD, Milowsky MI. 2017; A systematic review of strategies to prevent cisplatin-induced nephrotoxicity. Oncologist. 22:609–619. DOI: 10.1634/theoncologist.2016-0319. PMID: 28438887. PMCID: PMC5423518.
Article5. Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, Kalofonos H, Radulović S, Demey W, Ullén A, Loriot Y, idhar SS Sr, Tsuchiya N, Kopyltsov E, Sternberg CN, Bellmunt J, Aragon-Ching JB, Petrylak DP, Laliberte R, Wang J, et al. 2020; Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 383:1218–1230. DOI: 10.1056/NEJMoa2002788. PMID: 32945632.
Article6. Inman BA, Longo TA, Ramalingam S, Harrison MR. 2017; Atezolizumab: a PD-L1-blocking antibody for bladder cancer. Clin Cancer Res. 23:1886–1890. DOI: 10.1158/1078-0432.CCR-16-1417. PMID: 27903674.
Article7. Balar AV, Kamat AM, Kulkarni GS, Uchio EM, Boormans JL, Roumiguié M, Krieger LEM, Singer EA, Bajorin DF, Grivas P, Seo HK, Nishiyama H, Konety BR, Li H, Nam K, Kapadia E, Frenkl T, de Wit R. 2021; Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): an open-label, single-arm, multicentre, phase 2 study. Lancet Oncol. 22:919–930. Erratum in: Lancet Oncol. 2021;22:e347. DOI: 10.1016/S1470-2045(21)00147-9. PMID: 34051177.
Article8. Ellis SR, Vierra AT, Millsop JW, Lacouture ME, Kiuru M. 2020; Dermatologic toxicities to immune checkpoint inhibitor therapy: a review of histopathologic features. J Am Acad Dermatol. 83:1130–1143. DOI: 10.1016/j.jaad.2020.04.105. PMID: 32360716. PMCID: PMC7492441.
Article9. Rena G, Hardie DG, Pearson ER. 2017; The mechanisms of action of metformin. Diabetologia. 60:1577–1585. DOI: 10.1007/s00125-017-4342-z. PMID: 28776086. PMCID: PMC5552828.
Article10. Ugwueze CV, Ogamba OJ, Young EE, Onyenekwe BM, Ezeokpo BC. 2020; Metformin: a possible option in cancer chemotherapy. Anal Cell Pathol (Amst). 2020:7180923. DOI: 10.1155/2020/7180923. PMID: 32399389. PMCID: PMC7201450.
Article11. Peng M, Su Q, Zeng Q, Li L, Liu Z, Xue L, Cheng Y, Huang Y, Tao T, Lv H, Li X, Tao X, Guo P, Chen AF, Yang X. 2016; High efficacy of intravesical treatment of metformin on bladder cancer in preclinical model. Oncotarget. 7:9102–9117. DOI: 10.18632/oncotarget.6933. PMID: 26802022. PMCID: PMC4891029.
Article12. Flory J, Lipska K. 2019; Metformin in 2019. JAMA. 321:1926–1927. DOI: 10.1001/jama.2019.3805. PMID: 31009043. PMCID: PMC7552083.
Article13. Rosenthal PJ. 2008; Artesunate for the treatment of severe falciparum malaria. N Engl J Med. 358:1829–1836. DOI: 10.1056/NEJMct0709050. PMID: 18434652.
Article14. McDowell A Jr, Hill KS, McCorkle JR, Gorski J, Zhang Y, Salahudeen AA, Ueland F, Kolesar JM. 2021; Preclinical evaluation of artesunate as an antineoplastic agent in ovarian cancer treatment. Diagnostics (Basel). 11:395. DOI: 10.3390/diagnostics11030395. PMID: 33652561. PMCID: PMC7996621.
Article15. Niederreiter M, Klein J, Arndt K, Werner J, Mayer B. 2023; Anti-cancer effects of artesunate in human 3D tumor models of different complexity. Int J Mol Sci. 24:7844. DOI: 10.3390/ijms24097844. PMID: 37175551. PMCID: PMC10178545.
Article16. Singh A, Ruiz C, Bhalla K, Haley JA, Li QK, Acquaah-Mensah G, Montal E, Sudini KR, Skoulidis F, Wistuba II, Papadimitrakopoulou V, Heymach JV, Boros LG, Gabrielson E, Carretero J, Wong KK, Haley JD, Biswal S, Girnun GD. 2018; De novo lipogenesis represents a therapeutic target in mutant Kras non-small cell lung cancer. FASEB J. 32:fj201800204. DOI: 10.1096/fj.201800204. PMID: 29906244. PMCID: PMC6219836.
Article17. Röhrig F, Schulze A. 2016; The multifaceted roles of fatty acid synthesis in cancer. Nat Rev Cancer. 16:732–749. DOI: 10.1038/nrc.2016.89. PMID: 27658529.
Article18. Daneshmand S, Nazemi A. 2020; Neoadjuvant chemotherapy in variant histology bladder cancer: current evidence. Eur Urol Focus. 6:639–641. DOI: 10.1016/j.euf.2020.04.011. PMID: 32451316.
Article19. Broadfield LA, Pane AA, Talebi A, Swinnen JV, Fendt SM. 2021; Lipid metabolism in cancer: new perspectives and emerging mechanisms. Dev Cell. 56:1363–1393. DOI: 10.1016/j.devcel.2021.04.013. PMID: 33945792.
Article20. Menendez JA, Lupu R. 2007; Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 7:763–777. DOI: 10.1038/nrc2222. PMID: 17882277.
Article21. Lettieri Barbato D, Vegliante R, Desideri E, Ciriolo MR. 2014; Managing lipid metabolism in proliferating cells: new perspective for metformin usage in cancer therapy. Biochim Biophys Acta. 1845:317–324. DOI: 10.1016/j.bbcan.2014.02.003. PMID: 24569230.
Article22. Hoter A, Naim HY. 2019; Heat shock proteins and ovarian cancer: important roles and therapeutic opportunities. Cancers (Basel). 11:1389. DOI: 10.3390/cancers11091389. PMID: 31540420. PMCID: PMC6769485.
Article23. Deng J, Peng M, Zhou S, Xiao D, Hu X, Xu S, Wu J, Yang X. 2021; Metformin targets Clusterin to control lipogenesis and inhibit the growth of bladder cancer cells through SREBP-1c/FASN axis. Signal Transduct Target Ther. 6:98. DOI: 10.1038/s41392-021-00493-8. PMID: 33649289. PMCID: PMC7921554.
Article24. Zhang S, Li J, Nong X, Zhan Y, Xu J, Zhao D, Ma C, Wang Y, Li Y, Li Z, Li J. 2021; Artesunate combined with metformin ameliorate on diabetes-induced xerostomia by mitigating superior salivatory nucleus and salivary glands injury in type 2 diabetic rats via the PI3K/AKT pathway. Front Pharmacol. 12:774674. DOI: 10.3389/fphar.2021.774674. PMID: 34987398. PMCID: PMC8722737.
Article25. Ding W, Liao L, Liu J, Zhao J, Tang Q, Liao Y. 2023; Lower dose of metformin combined with artesunate induced autophagy-dependent apoptosis of glioblastoma by activating ROS-AMPK-mTOR axis. Exp Cell Res. 430:113691. DOI: 10.1016/j.yexcr.2023.113691. PMID: 37399981.
Article26. Würth R, Pattarozzi A, Gatti M, Bajetto A, Corsaro A, Parodi A, Sirito R, Massollo M, Marini C, Zona G, Fenoglio D, Sambuceti G, Filaci G, Daga A, Barbieri F, Florio T. 2013; Metformin selectively affects human glioblastoma tumor-initiating cell viability: A role for metformin-induced inhibition of Akt. Cell Cycle. 12:145–156. DOI: 10.4161/cc.23050. PMID: 23255107. PMCID: PMC3570504.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- YM155, specific survivin inhibitor, can enhance artesunate-induced cytotoxicity in HCT116 colon cancer cells
- Regulation of the Gene Expression of Airway MUC5AC Mucin through NF-κκB Signaling Pathway by Artesunate, an Antimalarial Agent
- Clusterin Expression and Apoptosis in Transitional Cell Carcinoma of the Bladder
- Is 5'-AMP-Activated Protein Kinase Both Jekyll and Hyde in Bladder Cancer?
- Artesunate Inhibits the Proliferation and Migration of Cutaneous Squamous Cell Carcinoma by Regulating the SLC7A11-GPX4 Pathway via the p300-p53 Axis