J Pathol Transl Med.
2024 May;58(3):117-126. 10.4132/jptm.2024.03.07.
Revisiting the utility of identifying nuclear grooves as unique nuclear changes by an object detector model
- Affiliations
-
- 1Bioregulation Department, Health and Science Institute, Federal University of Bahia, Salvador, Brazil
- 2Institute of Computing, Federal University of Bahia, Salvador, Brazil
- 3Institute of Biomedical Sciences, University of São Paulo, São Paulo, Brazil
- 4Endocrinology Department, Hospital de Braga, Braga, Portugal
- 5Laboratory of Pathology of the Institute of Pathology and Molecular Immunology, University of Porto, Porto, Portugal
- 6Department of Biomedical Genetics, University of Rochester, Rochester, New York, USA
- 7Faculty of Medicine, University of Porto, Porto, Portugal
- 8Postgraduate Program in Medicine and Health, Bahia Faculty of Medicine, Federal University of Bahia, Salvador, Brazil
- KMID: 2555537
- DOI: http://doi.org/10.4132/jptm.2024.03.07
Abstract
- Background
Among other structures, nuclear grooves are vastly found in papillary thyroid carcinoma (PTC). Considering that the application of artificial intelligence in thyroid cytology has potential for diagnostic routine, our goal was to develop a new supervised convolutional neural network capable of identifying nuclear grooves in Diff-Quik stained whole-slide images (WSI) obtained from thyroid fineneedle aspiration.
Methods
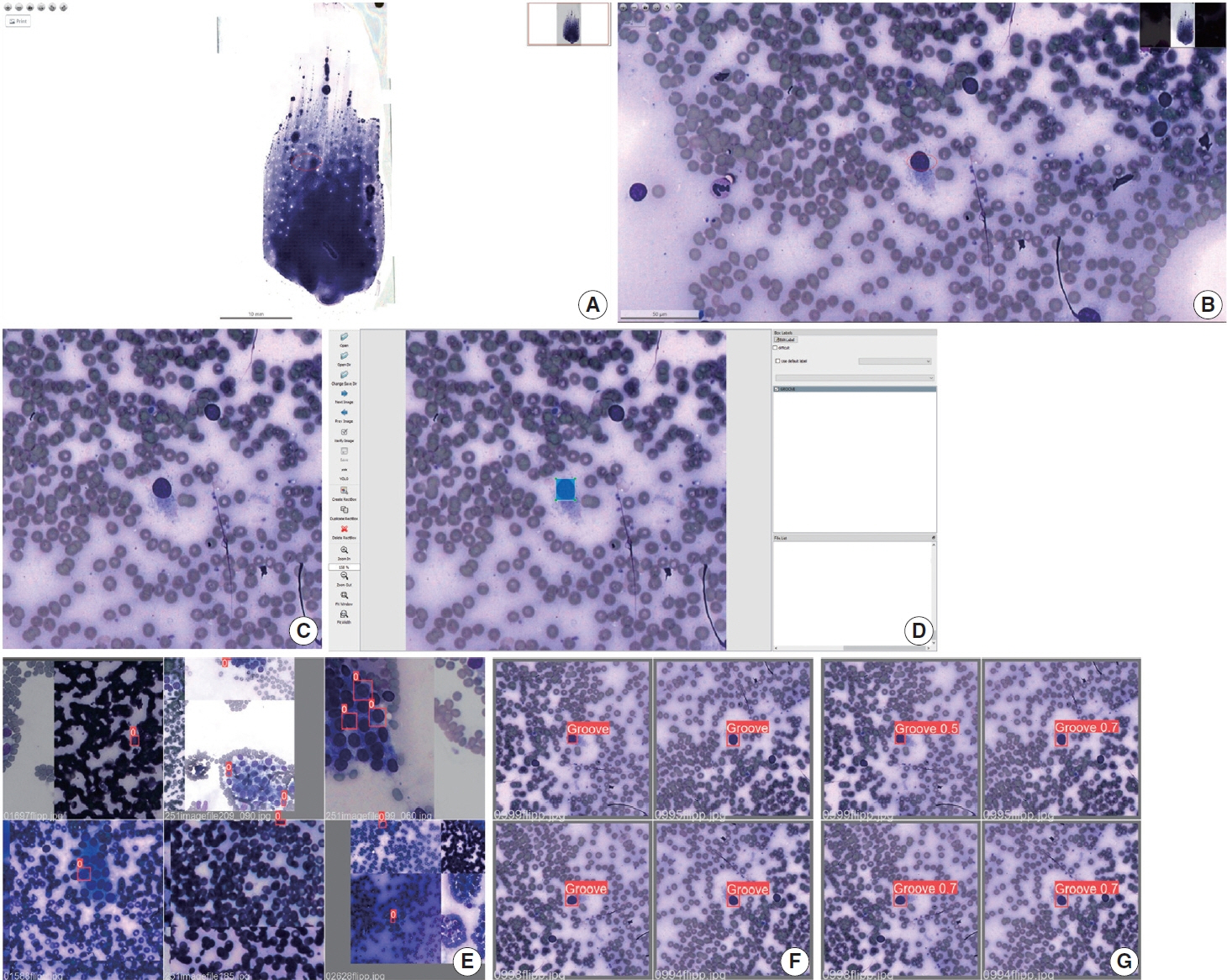
We selected 22 Diff-Quik stained cytological slides with cytological diagnosis of PTC and concordant histological diagnosis. Each of the slides was scanned, forming a WSI. Images that contained the region of interest were obtained, followed by pre-formatting, annotation of the nuclear grooves and data augmentation techniques. The final dataset was divided into training and validation groups in a 7:3 ratio.
Results
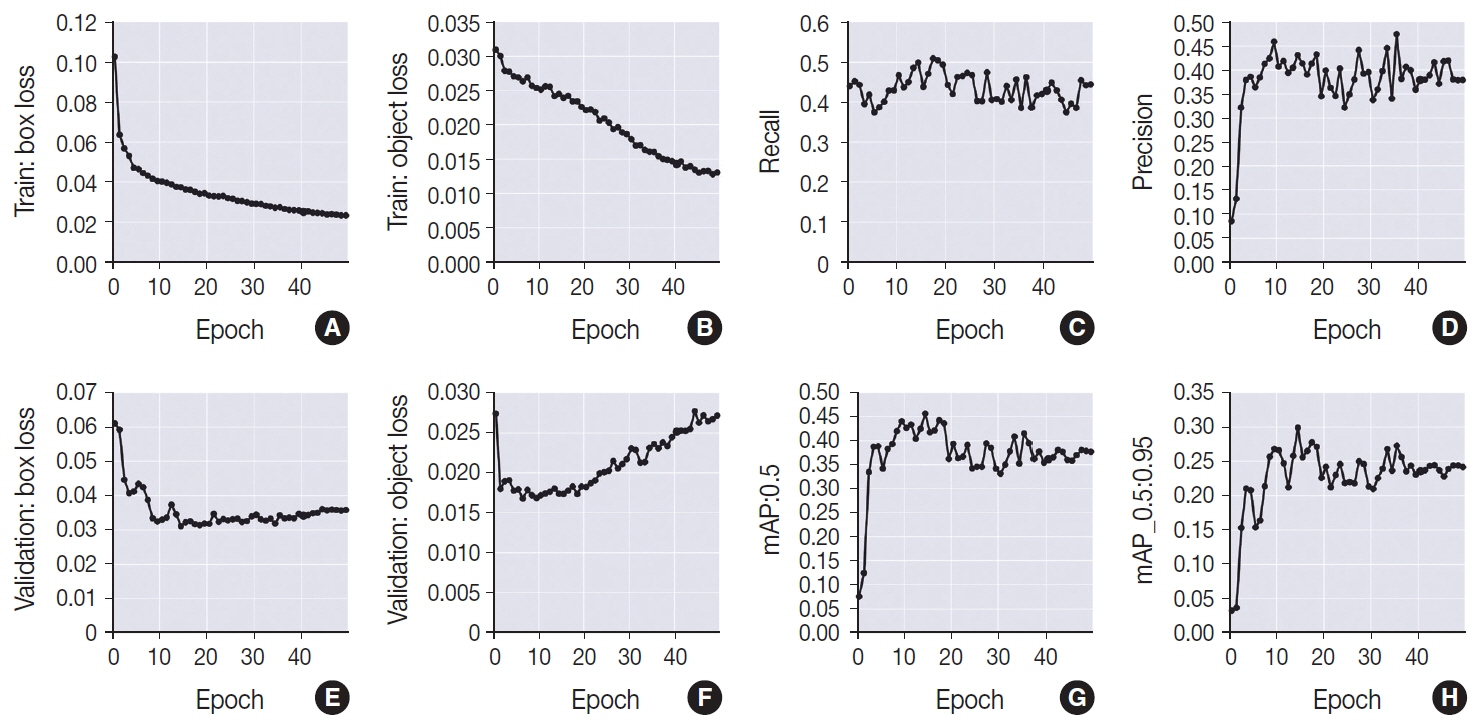
This is the first artificial intelligence model based on object detection applied to nuclear structures in thyroid cytopathology. A total of 7,255 images were obtained from 22 WSI, totaling 7,242 annotated nuclear grooves. The best model was obtained after it was submitted 15 times with the train dataset (14th epoch), with 67% true positives, 49.8% for sensitivity and 43.1% for predictive positive value.
Conclusions
The model was able to develop a structure predictor rule, indicating that the application of an artificial intelligence model based on object detection in the identification of nuclear grooves is feasible. Associated with a reduction in interobserver variability and in time per slide, this demonstrates that nuclear evaluation constitutes one of the possibilities for refining the diagnosis through computational models.
Figure
Reference
-
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.
Article2. Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006; 295:2164–7.
Article3. Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol. 2013; 2013:965212.
Article4. Mora-Guzman I, Munoz de Nova JL, Marin-Campos C, et al. Efficiency of the Bethesda System for Thyroid Cytopathology. Cir Esp (Engl Ed). 2018; 96:363–8.
Article5. Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med. 2016; 375:614–7.
Article6. Daskalakis A, Kostopoulos S, Spyridonos P, et al. Design of a multiclassifier system for discriminating benign from malignant thyroid nodules using routinely H&E-stained cytological images. Comput Biol Med. 2008; 38:196–203.
Article7. Chain K, Legesse T, Heath JE, Staats PN. Digital image-assisted quantitative nuclear analysis improves diagnostic accuracy of thyroid fine-needle aspiration cytology. Cancer Cytopathol. 2019; 127:501–13.
Article8. Gerhard R, Teixeira S, Gaspar da Rocha A, Schmitt F. Thyroid fineneedle aspiration cytology: is there a place to virtual cytology? Diagn Cytopathol. 2013; 41:793–8.
Article9. Fragopoulos C, Pouliakis A, Meristoudis C, et al. Radial basis function artificial neural network for the investigation of thyroid cytological lesions. J Thyroid Res. 2020; 2020:5464787.
Article10. Schmitt WR. Punção aspirativa por agulha fina e a sua importância diagnóstica nas lesões de tireoide [Fine needle aspiration and its diagnostic importance in thyroid lesions]. Porto: Universidade do Porto;2011.11. Shurbaji MS, Gupta PK, Frost JK. Nuclear grooves: a useful criterion in the cytopathologic diagnosis of papillary thyroid carcinoma. Diagn Cytopathol. 1988; 4:91–4.
Article12. Ali SZ, Cibas ES. The Bethesda System for Reporting Thyroid Cytopathology. Cham: Springer;2018.13. Cibas ES, Ali SZ. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2017; 27:1341–6.
Article14. Ali SZ, VanderLaan PA. The Bethesda System for Reporting Thyroid Cytopathology. Cham: Springer;2023.15. LiVolsi VA. Papillary thyroid carcinoma: an update. Mod Pathol. 2011; 24 Suppl 2:S1–9.
Article16. Baloch ZW, LiVolsi VA, Asa SL, et al. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008; 36:425–37.
Article17. Batistatou A, Scopa CD. Pathogenesis and diagnostic significance of nuclear grooves in thyroid and other sites. Int J Surg Pathol. 2009; 17:107–10.
Article18. Francis IM, Das DK, Sheikh ZA, Sharma PN, Gupta SK. Role of nuclear grooves in the diagnosis of papillary thyroid carcinoma: a quantitative assessment on fine needle aspiration smears. Acta Cytol. 1995; 39:409–15.19. Gould E, Watzak L, Chamizo W, Albores-Saavedra J. Nuclear grooves in cytologic preparations: a study of the utility of this feature in the diagnosis of papillary carcinoma. Acta Cytol. 1989; 33:16–20.20. Das DK. Intranuclear cytoplasmic inclusions in fine-needle aspiration smears of papillary thyroid carcinoma: a study of its morphological forms, association with nuclear grooves, and mode of formation. Diagn Cytopathol. 2005; 32:264–8.
Article21. Yang YJ, Demirci SS. Evaluating the diagnostic significance of nuclear grooves in thyroid fine needle aspirates with a semiquantitative approach. Acta Cytol. 2003; 47:563–70.22. Rupp M, Ehya H. Nuclear grooves in the aspiration cytology of papillary carcinoma of the thyroid. Acta Cytol. 1989; 33:21–6.23. Scopa CD, Melachrinou M, Saradopoulou C, Merino MJ. The significance of the grooved nucleus in thyroid lesions. Mod Pathol. 1993; 6:691–4.24. Silverman JF, Frable WJ. The use of the diff-quik stain in the immediate interpretation of fine-needle aspiration biopsies. Diagn Cytopathol. 1990; 6:366–9.
Article25. Dey P. Basic and advanced laboratory techniques in histopathology and cytology. Singapore: Springer Singapore;2018.26. Bhambhani S, Kashyap V, Das DK. Nuclear grooves. Valuable diagnostic feature in May-Grunwald-Giemsa-stained fine needle aspirates of papillary carcinoma of the thyroid. Acta Cytol. 1990; 34:809–12.27. Niazi MKK, Parwani AV, Gurcan MN. Digital pathology and artificial intelligence. Lancet Oncol. 2019; 20:e253–61.
Article28. Bera K, Schalper KA, Rimm DL, Velcheti V, Madabhushi A. Artificial intelligence in digital pathology: new tools for diagnosis and precision oncology. Nat Rev Clin Oncol. 2019; 16:703–15.
Article29. Pouliakis A, Karakitsou E, Margari N, et al. Artificial neural networks as decision support tools in cytopathology: past, present, and future. Biomed Eng Comput Biol. 2016; 7:1–18.
Article30. Gupta N, Sarkar C, Singh R, Karak AK. Evaluation of diagnostic efficiency of computerized image analysis based quantitative nuclear parameters in papillary and follicular thyroid tumors using paraffin-embedded tissue sections. Pathol Oncol Res. 2001; 7:46–55.
Article31. Valentim FO, Coelho BP, Miot HA, et al. Follicular thyroid lesions: is there a discriminatory potential in the computerized nuclear analysis? Endocr Connect. 2018; 7:907–13.
Article32. Yashaswini R, Suresh TN, Sagayaraj A. Cytological evaluation of thyroid lesions by nuclear morphology and nuclear morphometry. J Cytol. 2017; 34:197–202.
Article33. Karakitsos P, Cochand-Priollet B, Guillausseau PJ, Pouliakis A. Potential of the back propagation neural network in the morphologic examination of thyroid lesions. Anal Quant Cytol Histol. 1996; 18:494–500.34. Ramos HE, Vale J, Lopes S, et al. Nuclear score evaluation in follicular-patterned thyroid lesions using optical and digital environments. Endocrine. 2022; 77:486–92.
Article35. Kezlarian B, Lin O. Artificial intelligence in thyroid fine needle aspiration biopsies. Acta Cytol. 2021; 65:324–9.
Article36. Legesse T, Parker L, Heath J, Staats PN. Distinguishing non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) from classic and invasive follicular-variant papillary thyroid carcinomas based on cytologic features. J Am Soc Cytopathol. 2019; 8:11–7.
Article37. Kuzan TY, Guzelbey B, Turan Guzel N, Kuzan BN, Cakir MS, Canbey C. Analysis of intra-observer and inter-observer variability of pathologists for non-benign thyroid fine needle aspiration cytology according to Bethesda system categories. Diagn Cytopathol. 2021; 49:850–5.
Article38. Cibas ES, Baloch ZW, Fellegara G, et al. A prospective assessment defining the limitations of thyroid nodule pathologic evaluation. Ann Intern Med. 2013; 159:325–32.
Article39. Thompson LD, Poller DN, Kakudo K, Burchette R, Nikiforov YE, Seethala RR. An international interobserver variability reporting of the nuclear scoring criteria to diagnose noninvasive follicular thyroid neoplasm with papillary-like nuclear features: a validation study. Endocr Pathol. 2018; 29:242–9.
Article40. Liu Z, Bychkov A, Jung CK, et al. Interobserver and intraobserver variation in the morphological evaluation of noninvasive follicular thyroid neoplasm with papillary-like nuclear features in Asian practice. Pathol Int. 2019; 69:202–10.
Article41. House JC, Henderson-Jackson EB, Johnson JO, et al. Diagnostic digital cytopathology: are we ready yet? J Pathol Inform. 2013; 4:28.
Article42. Vodovnik A. Diagnostic time in digital pathology: a comparative study on 400 cases. J Pathol Inform. 2016; 7:4.
Article43. Jiang P, Ergu D, Liu F, Cai Y, Ma B. A review of Yolo algorithm developments. Procedia Comput Sci. 2022; 199:1066–73.
Article44. Sanyal P, Mukherjee T, Barui S, Das A, Gangopadhyay P. Artificial intelligence in cytopathology: a neural network to identify papillary carcinoma on thyroid fine-needle aspiration cytology smears. J Pathol Inform. 2018; 9:43.
Article45. Guan Q, Wang Y, Ping B, et al. Deep convolutional neural network VGG-16 model for differential diagnosing of papillary thyroid carcinomas in cytological images: a pilot study. J Cancer. 2019; 10:4876–82.
Article46. Duan W, Gao L, Liu J, et al. Computer-assisted fine-needle aspiration cytology of thyroid using two-stage refined convolutional neural network. Electronics. 2022; 11:4089.
Article47. Nguyen DUC, Lee YM, Park J. An Ensemble deep learning for automatic prediction of papillary thyroid carcinoma using fine needle aspiration cytology. Expert Syst Appl. 2021; 188:115927.48. Aloqaily A, Polonia A, Campelos S, et al. Digital versus optical diagnosis of follicular patterned thyroid lesions. Head Neck Pathol. 2021; 15:537–43.
Article