Korean J Pain.
2024 Apr;37(2):141-150. 10.3344/kjp.23318.
Evaluation of the antinociceptive activities of natural propolis extract derived from stingless bee Trigona thoracica in mice
- Affiliations
-
- 1Faculty of Medicine, Universiti Sultan Zainal Abidin, Kuala Terengganu, Malaysia
- 2Department of Anaesthesiology and Intensive Care, Hospital Pengajar Universiti Sultan Zainal Abidin, Kuala Nerus, Malaysia
- 3Department of Anesthesiology and Pain Medicine, Seoul National University Hospital, Seoul, Korea
- 4Department of Anesthesiology and Pain Medicine, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2554954
- DOI: http://doi.org/10.3344/kjp.23318
Abstract
- Background
Stingless bee propolis is a popular traditional folk medicine and has been employed since ancient times. This study aimed to evaluate the antinociceptive activities of the chemical constituents of aqueous propolis extract (APE) collected by Trigona thoracica in a nociceptive model in mice.
Methods
The identification of chemical constituents of APE was performed using high-performance liquid chromatography (HPLC). Ninety-six male Swiss mice were administered APE (400 mg/kg, 1,000 mg/kg, and 2,000 mg/kg) before developing nociceptive pain models. Then, the antinociceptive properties of each APE dose were evaluated in acetic acid-induced abdominal constriction, hot plate test, and formalin-induced paw licking test. Administration of normal saline, acetylsalicylic acid (ASA, 100 mg/kg, orally), and morphine (5 mg/kg, intraperitoneally) were used for the experiments.
Results
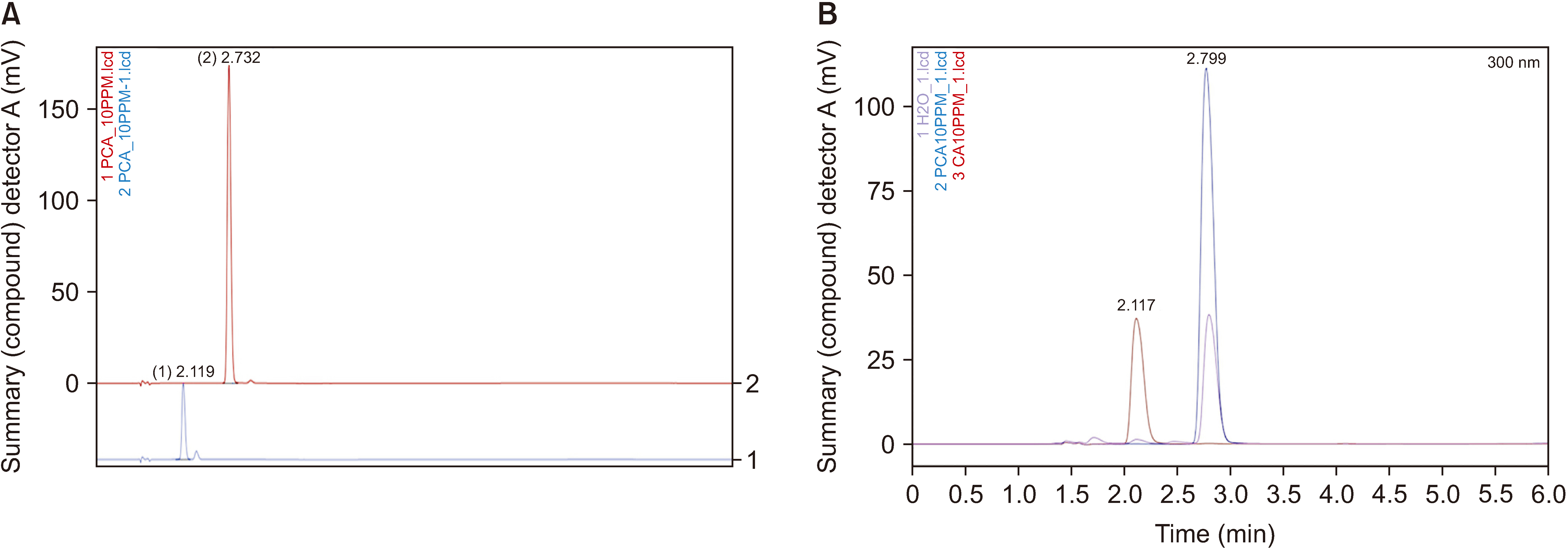
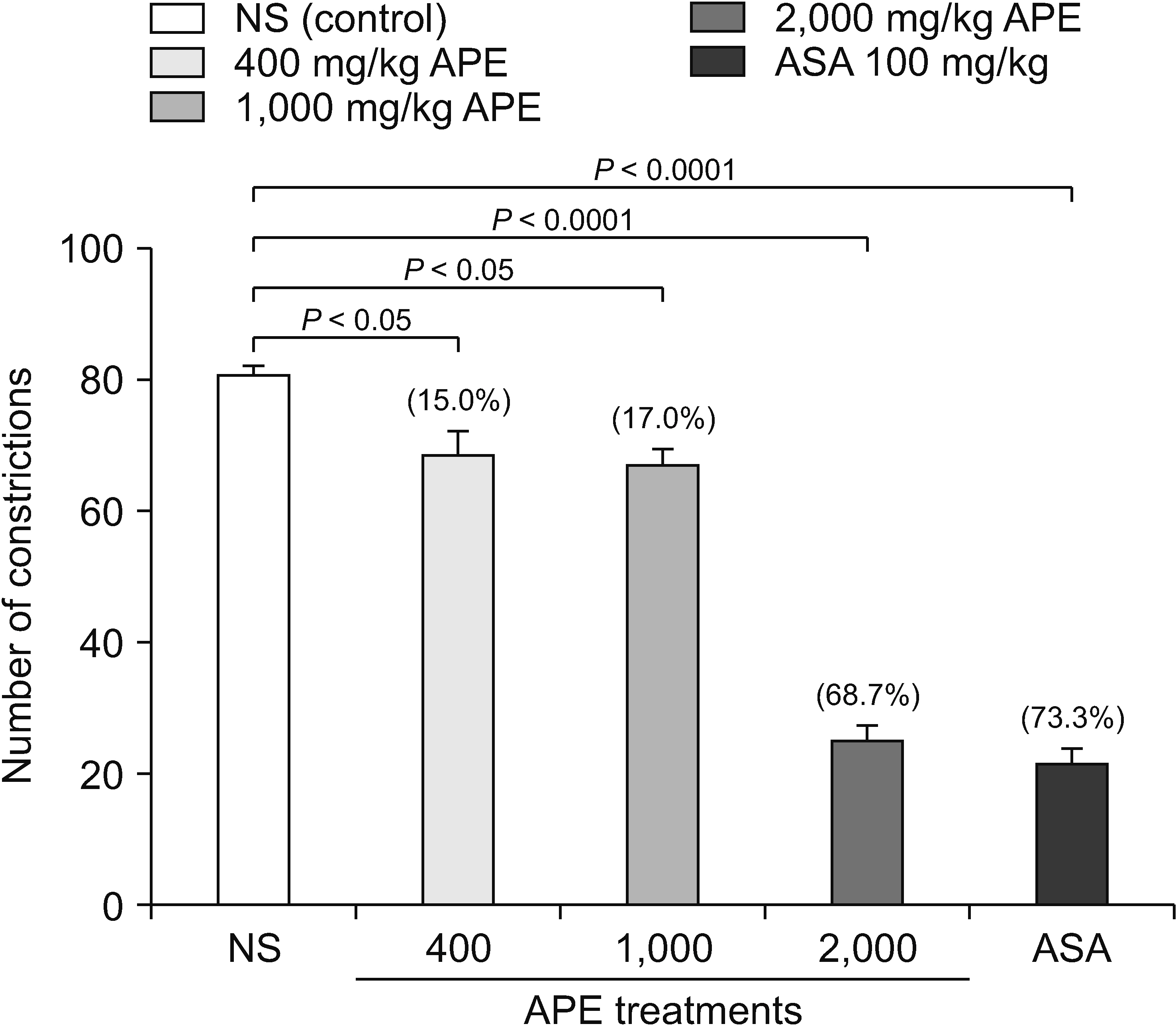
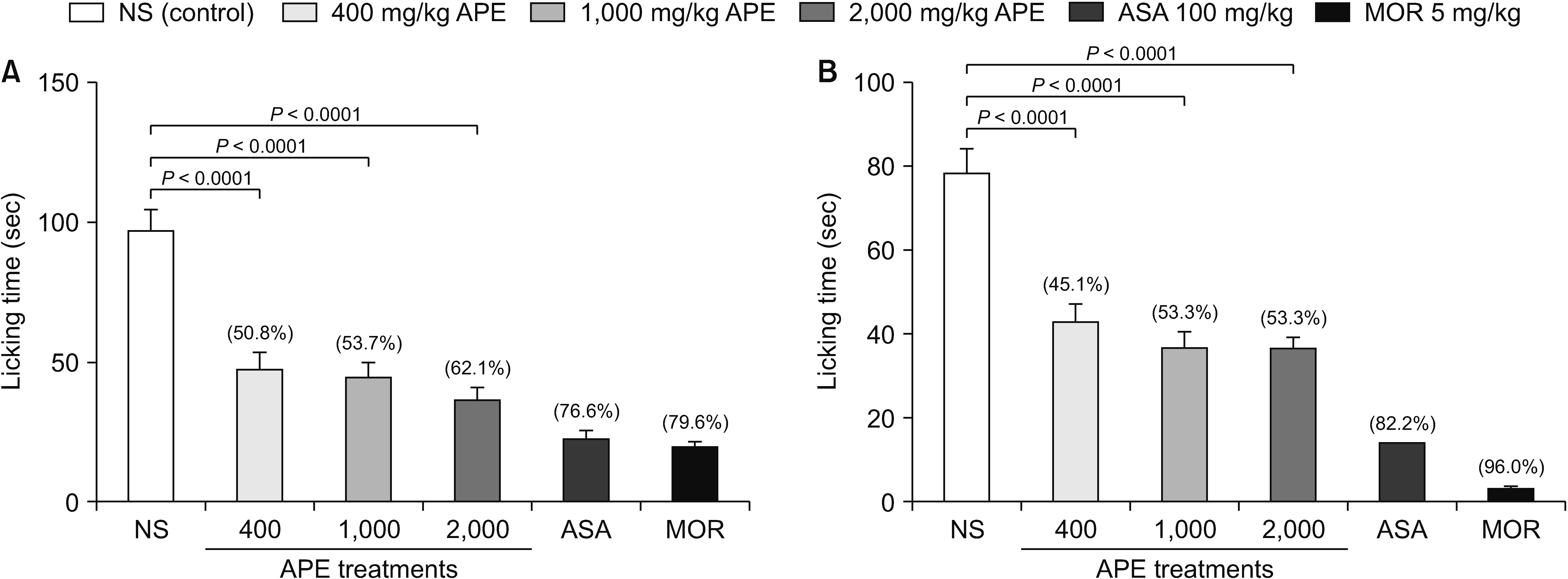
HPLC revealed that the APE from Trigona thoracica contained p-coumaric acid (R2 = 0.999) and caffeic acid (R2 = 0.998). Although all APE dosages showed inhibition of acetic acid-induced abdominal constriction, only 2,000 mg/kg was comparable to the result of ASA (68.7% vs. 73.3%, respectively). In the hot plate test, only 2,000 mg/ kg of APE increased the latency time significantly compared to the control. In the formalin test, the durations of paw licking were significantly reduced at early and late phases in all APE groups with a decrease from 45.1% to 53.3%.
Conclusions
APE from Trigona thoracica, containing p-coumaric acid and caffeic acid, exhibited antinociceptive effects, which supports its potential use in targeting the prevention or reversal of central and peripheral sensitization that may produce clinical pain conditions.
Keyword
Figure
Reference
-
1. Heard TA. 1999; The role of stingless bees in crop pollination. Annu Rev Entomol. 44:183–206. DOI: 10.1146/annurev.ento.44.1.183. PMID: 15012371.
Article2. Ruttner F. Ruttner F, editor. 1988. Stingless bees (Meliponinae). In: Biogeography and taxonomy of honeybees. Springer;Berlin, Heidelberg: p. 13–9. DOI: 10.1007/978-3-642-72649-1_2.
Article3. Kelly N, Farisya MSN, Kumara TK, Marcela P. 2014; Species diversity and external nest characteristics of stingless bees in meliponiculture. Pertanika J Trop Agric Sci. 37:293–8.4. Campos JF, dos Santos UP, Macorini LF, de Melo AM, Balestieri JB, Paredes-Gamero EJ, et al. 2014; Antimicrobial, antioxidant and cytotoxic activities of propolis from Melipona orbignyi (Hymenoptera, Apidae). Food Chem Toxicol. 65:374–80. DOI: 10.1016/j.fct.2014.01.008. PMID: 24412556.
Article5. Campos JF, Dos Santos UP, da Rocha Pdos S, Damião MJ, Balestieri JB, Cardoso CA, et al. 2015; Antimicrobial, antioxidant, anti-inflammatory, and cytotoxic activities of propolis from the stingless bee Tetragonisca fiebrigi (Jataí). Evid Based Complement Alternat Med. 2015:296186. DOI: 10.1155/2015/296186. PMID: 26185516. PMCID: PMC4491730.6. Torres AR, Sandjo LP, Friedemann MT, Tomazzoli MM, Maraschin M, Mello CF, et al. 2018; Chemical characterization, antioxidant and antimicrobial activity of propolis obtained from Melipona quadrifasciata quadrifasciata and Tetragonisca angustula stingless bees. Braz J Med Biol Res. 51:e7118. DOI: 10.1590/1414-431x20187118. PMID: 29791598. PMCID: PMC6002130.
Article7. Machado JL, Assunção AK, da Silva MC, Dos Reis AS, Costa GC, Arruda Dde S, et al. 2012; Brazilian green propolis: anti-inflammatory property by an immunomodulatory activity. Evid Based Complement Alternat Med. 2012:157652. DOI: 10.1155/2012/157652. PMID: 23320022. PMCID: PMC3541042.
Article8. Touzani S, Embaslat W, Imtara H, Kmail A, Kadan S, Zaid H, et al. 2019; In vitro evaluation of the potential use of propolis as a multitarget therapeutic product: physicochemical properties, chemical composition, and immunomodulatory, antibacterial, and anticancer properties. Biomed Res Int. 2019:4836378. DOI: 10.1155/2019/4836378. PMID: 31915694. PMCID: PMC6930758.9. Ismail TNNT, Sulaiman SA, Ponnuraj KT, Man CN, Hassan NB. 2018; Chemical constituents of Malaysian Apis mellifera propolis. Sains Malaysiana. 47:117–22. DOI: 10.17576/jsm-2018-4701-14.10. Mohamed WAS, Ismail NZ, Omar EA, Abdul Samad N, Adam SK, Mohamad S. 2020; GC-MS evaluation, antioxidant content, and cytotoxic activity of propolis extract from Peninsular Malaysian stingless bees, Tetrigona Apicalis. Evid Based Complement Alternat Med. 2020:8895262. DOI: 10.1155/2020/8895262. PMID: 33381215. PMCID: PMC7759394.11. Ibrahim N, Mohd Niza NFS, Mohd Rodi MM, Zakaria AJ, Ismail Z, Mohd KS. 2016; Chemical and biological analyses of Malaysian stingless bee propolis extracts. MJAS. 20:413–22. DOI: 10.17576/mjas-2016-2002-26.
Article12. Blakemore PR, White JD. 2002; Morphine, the Proteus of organic molecules. Chem Commun. 2:1159–68. DOI: 10.1039/b111551k. PMID: 12109065.
Article13. Allison MC, Howatson AG, Torrance CJ, Lee FD, Russell RI. 1992; Gastrointestinal damage associated with the use of nonsteroidal antiinflammatory drugs. N Engl J Med. 327:749–54. DOI: 10.1056/NEJM199209103271101. PMID: 1501650.
Article14. Brodkiewicz Y, Marcinkevicius K, Reynoso M, Salomon V, Maldonado L, Vera N. 2018; Studies of the biological and therapeutic effects of Argentine stingless bee propolis. JDDT. 8:382–92. DOI: 10.22270/jddt.v8i5.1889.
Article15. Al-Hariri MT, Abualait TS. 2020; Effects of green Brazilian propolis alcohol extract on nociceptive pain models in rats. Plants (Basel). 9:1102. DOI: 10.3390/plants9091102. PMID: 32867097. PMCID: PMC7570148.
Article16. Sun L, Liao L, Wang B. 2018; Potential antinociceptive effects of Chinese propolis and identification on its active compounds. J Immunol Res. 2018:5429543. DOI: 10.1155/2018/5429543. PMID: 30356413. PMCID: PMC6178491.
Article17. Tiveron AP, Rosalen PL, Franchin M, Lacerda RC, Bueno-Silva B, Benso B, et al. 2016; Chemical characterization and antioxidant, antimicrobial, and anti-inflammatory activities of South Brazilian organic propolis. PLoS One. 11:e0165588. DOI: 10.1371/journal.pone.0165588. PMID: 27802316. PMCID: PMC5089781.
Article18. Lima Cavendish R, de Souza Santos J, Belo Neto R, Oliveira Paixão A, Valéria Oliveira J, Divino de Araujo E, et al. 2015; Antinociceptive and anti-inflammatory effects of Brazilian red propolis extract and formononetin in rodents. J Ethnopharmacol. 173:127–33. DOI: 10.1016/j.jep.2015.07.022. PMID: 26192808.
Article19. Aziz MSA, Giribabu N, Rao PV, Salleh N. 2017; Pancreatoprotective effects of Geniotrigona thoracica stingless bee honey in streptozotocin-nicotinamide-induced male diabetic rats. Biomed Pharmacother. 89:135–45. DOI: 10.1016/j.biopha.2017.02.026. PMID: 28222394.
Article20. Abdullah NA, Zullkiflee N, Zaini SNZ, Taha H, Hashim F, Usman A. 2020; Phytochemicals, mineral contents, antioxidants, and antimicrobial activities of propolis produced by Brunei stingless bees Geniotrigona thoracica, Heterotrigona itama, and Tetrigona binghami. Saudi J Biol Sci. 27:2902–11. DOI: 10.1016/j.sjbs.2020.09.014. PMID: 33100845. PMCID: PMC7569112.21. Colucci M, Maione F, Bonito MC, Piscopo A, Di Giannuario A, Pieretti S. 2008; New insights of dimethyl sulphoxide effects (DMSO) on experimental in vivo models of nociception and inflammation. Pharmacol Res. 57:419–25. DOI: 10.1016/j.phrs.2008.04.004. PMID: 18508278.
Article22. Mohd Salim NH, Azam Omar E, Wan Omar WA, Mohamed R. 2018; Chemical constituents and antioxidant activity of ethanolic extract of propolis from Malaysian stingless bee Geniotrigona thoracica species. Res J Pharm Biol Chem Sci. 9:646–51.23. Sani MH, Zakaria ZA, Balan T, Teh LK, Salleh MZ. 2012; Antinociceptive activity of methanol extract of Muntingia calabura leaves and the mechanisms of action involved. Evid Based Complement Alternat Med. 2012:890361. DOI: 10.1155/2012/890361. PMID: 22611437. PMCID: PMC3351243.24. Hajhashemi V, Khodarahmi G, Asadi P, Rajabi H. 2022; Evaluation of the antinociceptive effects of a selection of triazine derivatives in mice. Korean J Pain. 35:440–6. DOI: 10.3344/kjp.2022.35.4.440. PMID: 36175343. PMCID: PMC9530681.
Article25. Muhamad Suhaini NA, Pauzi MF, Juhari SN, Mohd KS, Abu Bakar NA. 2023; In vivo toxicity study on the effects of aqueous propolis extract from Malaysian stingless bee (Geniotrigona thoracica) in mice. Malays Appl Biol. 52:61–9. DOI: 10.55230/mabjournal.v52i2.2646.
Article26. Ramos AFN, Miranda JL. 2007; Propolis: a review of its anti-inflammatory and healing actions. J Venom Anim Toxins incl Trop Dis. 13:697–710. DOI: 10.1590/S1678-91992007000400002.
Article27. Zhu W, Chen M, Shou Q, Li Y, Hu F. 2011; Biological activities of Chinese propolis and Brazilian propolis on streptozotocin-induced type 1 diabetes mellitus in rats. Evid Based Complement Alternat Med. 2011:468529. DOI: 10.1093/ecam/neq025. PMID: 21785625. PMCID: PMC3135653.
Article28. Olczyk P, Ramos P, Komosinska-Vassev K, Stojko J, Pilawa B. 2013; Positive effect of propolis on free radicals in burn wounds. Evid Based Complement Alternat Med. 2013:356737. DOI: 10.1155/2013/356737. PMID: 23762125. PMCID: PMC3676959.
Article29. Kasote DM, Pawar MV, Gundu SS, Bhatia R, Nandre VS, Jagtap SD, et al. 2019; Chemical profiling, antioxidant, and antimicrobial activities of Indian stingless bees propolis samples. J Apic Res. 58:617–25. DOI: 10.1080/00218839.2019.1584960.
Article30. Bankova VS, Trusheva B, Popova M. 2021; Propolis extraction methods: a review. J Apic Res. 60:734–43. DOI: 10.1080/00218839.2021.1901426.
Article31. Park SH, Sim YB, Kim SM, Lee JK, Jung JS, Suh HW. 2011; The effect of caffeic acid on the antinociception and mechanisms in mouse. J Appl Biol Chem. 54:177–82. DOI: 10.3839/jksabc.2011.029.
Article32. Vogel HG. 2007. Drug discovery and evaluation: pharmacological assays. Heidelberg;Springer Berlin: DOI: 10.1007/978-3-540-70995-4.33. Le Bars D, Gozariu M, Cadden SW. 2001; Animal models of nociception. Pharmacol Rev. 53:597–652.34. Akindele AJ, Ibe IF, Adeyemi OO. 2011; Analgesic and antipyretic activities of Drymaria cordata (Linn.) Willd (Caryophyllaceae) extract. Afr J Tradit Complement Altern Med. 9:25–35. DOI: 10.4314/ajtcam.v9i1.4. PMID: 23983316. PMCID: PMC3746538.35. Zakaria ZA, Kumar GH, Mat Jais AM, Sulaiman MR, Somchit MN. 2008; Antinociceptive, antiinflammatory and antipyretic properties of Channa striatus fillet aqueous and lipid-based extracts in rats. Methods Find Exp Clin Pharmacol. 30:355–62. DOI: 10.1358/mf.2008.30.5.1236620. PMID: 18806894.
Article36. Franchin M, da Cunha MG, Denny C, Napimoga MH, Cunha TM, Koo H, et al. 2012; Geopropolis from Melipona scutellaris decreases the mechanical inflammatory hypernociception by inhibiting the production of IL-1β and TNF-α. J Ethnopharmacol. 143:709–15. DOI: 10.1016/j.jep.2012.07.040. PMID: 22885134.
Article37. McDonald J, Lambert DG. 2005; Opioid receptors. Cont Educ Anaesth Crit Care Pain. 5:22–5. DOI: 10.1093/bjaceaccp/mki004.
Article38. Vidyalakshmi K, Kamalakannan P, Viswanathan S, Ramaswamy S. 2010; Antinociceptive effect of certain dihydroxy flavones in mice. Pharmacol Biochem Behav. 96:1–6. DOI: 10.1016/j.pbb.2010.03.010. PMID: 20346369.
Article39. Mountassir M, Chaib S, Selami Y, Khalki H, Ouachrif A, Moubtakir S, et al. 2014; Antinociceptive activity and acute toxicity of Moroccan black propolis. IJERT. 3:2393–7.40. Tjølsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. 1992; The formalin test: an evaluation of the method. Pain. 51:5–17. DOI: 10.1016/0304-3959(92)90003-T. PMID: 1454405.
Article41. Hunskaar S, Hole K. 1987; The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 30:103–14. DOI: 10.1016/0304-3959(87)90088-1. PMID: 3614974.
Article42. Shibata M, Ohkubo T, Takahashi H, Inoki R. 1989; Modified formalin test: characteristic biphasic pain response. Pain. 38:347–52. DOI: 10.1016/0304-3959(89)90222-4. PMID: 2478947.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Propolis and Caffeic Acid Phenethyl Ester on Tumorigenesis, Pulmonary Metastases, and Activities of Splenocytes and Macrophages in Mice
- A Case of Allergic Contact Dermatitis by Propolis
- In vitro Evaluation of Antidermatophytic Activity of Egyptian Bee Propolis in Combination with Plant Essential Oils in Sheep Hoof Plate: An Experimental Model
- Systemic Contact Dermatitis from Propolis Ingestion
- In vitro Evaluation of the Antifungal Activity of Propolis Extract on Cryptococcus neoformans and Candida albicans