Endocrinol Metab.
2024 Apr;39(2):324-333. 10.3803/EnM.2023.1885.
Clinicopathological Features and Molecular Signatures of Lateral Neck Lymph Node Metastasis in Papillary Thyroid Microcarcinoma
- Affiliations
-
- 1CHA University School of Medicine, Seongnam, Korea
- 2Department of Internal Medicine, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul, Korea
- 3Department of Pathology, CHA Bundang Medical Center, CHA University School of Medicine, Seongnam, Korea
- KMID: 2554637
- DOI: http://doi.org/10.3803/EnM.2023.1885
Abstract
- Background
The predictive factors for lateral neck lymph node metastasis (LLNM) in papillary thyroid microcarcinoma (PTMC) remain undetermined. This study investigated the clinicopathological characteristics, transcriptomes, and tumor microenvironment in PTMC according to the LLNM status. We aimed to identify the biomarkers associated with LLNM development.
Methods
We retrospectively reviewed the medical records of patients with PTMC from two independent institutions between 2018 and 2022 (n=597 and n=467). We compared clinicopathological features between patients without lymph node metastasis (N0) and those with LLNM (N1b). Additionally, laser capture microdissection and RNA sequencing were performed on primary tumors from both groups, including metastatic lymph nodes from the N1b group (n=30; 20 primary tumors and 10 paired LLNMs). We corroborated the findings using RNA sequencing data from 16 BRAF-like PTMCs from The Cancer Genome Atlas. Transcriptomic analyses were validated by immunohistochemical staining.
Results
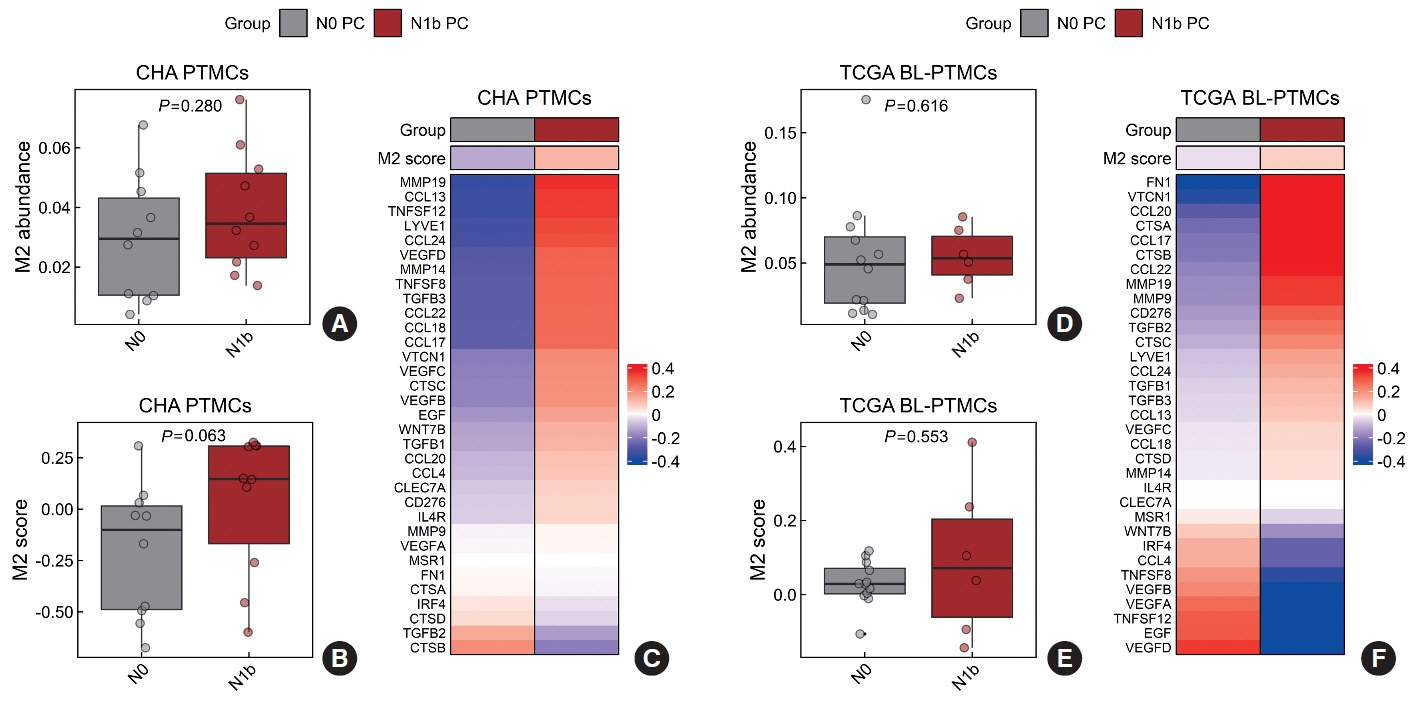
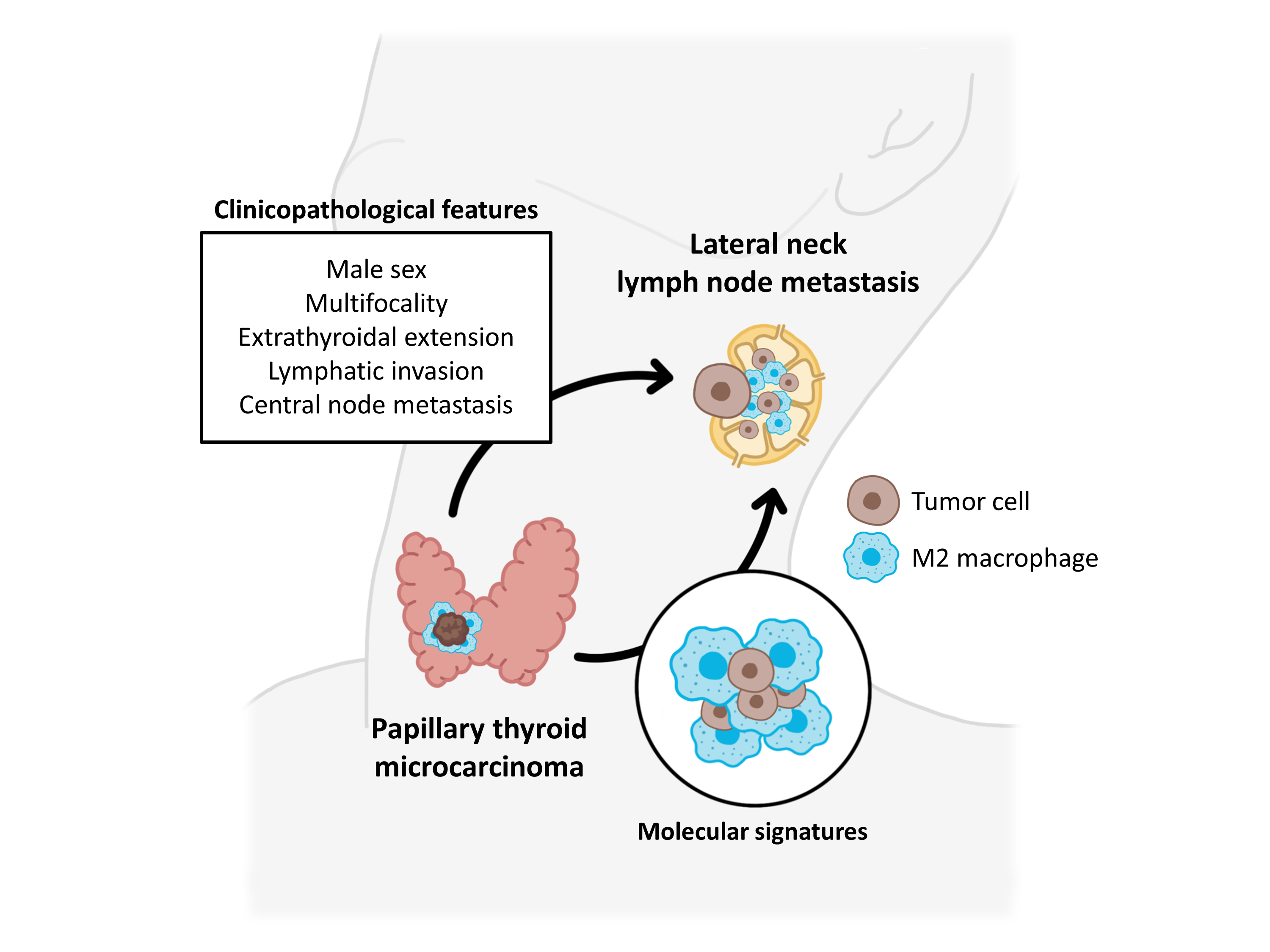
Clinicopathological characteristics, such as male sex, multifocality, extrathyroidal extension, lymphatic invasion, and central node metastasis showed associations with LLNM in PTMCs. Transcriptomic profiles between the N0 and N1b PTMC groups were similar. However, tumor microenvironment deconvolution from RNA sequencing and immunohistochemistry revealed an increased abundance of tumor-associated macrophages, particularly M2 macrophages, in the N1b group.
Conclusion
Patients with PTMC who have a male sex, multifocality, extrathyroidal extension, lymphatic invasion, and central node metastasis exhibited an elevated risk for LLNM. Furthermore, infiltration of M2 macrophages in the tumor microenvironment potentially supports tumor progression and LLNM in PTMCs.
Figure
Reference
-
1. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133.
Article2. Yi KH. The revised 2016 Korean Thyroid Association guidelines for thyroid nodules and cancers: differences from the 2015 American Thyroid Association guidelines. Endocrinol Metab (Seoul). 2016; 31:373–8.
Article3. Filetti S, Durante C, Hartl D, Leboulleux S, Locati LD, Newbold K, et al. Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019; 30:1856–83.
Article4. Sugitani I, Ito Y, Takeuchi D, Nakayama H, Masaki C, Shindo H, et al. Indications and strategy for active surveillance of adult low-risk papillary thyroid microcarcinoma: consensus statements from the Japan Association of Endocrine Surgery Task Force on management for papillary thyroid microcarcinoma. Thyroid. 2021; 31:183–92.
Article5. Haddad RI, Bischoff L, Ball D, Bernet V, Blomain E, Busaidy NL, et al. Thyroid carcinoma, version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022; 20:925–51.6. Wada N, Duh QY, Sugino K, Iwasaki H, Kameyama K, Mimura T, et al. Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann Surg. 2003; 237:399–407.7. Kim YS. Patterns and predictive factors of lateral lymph node metastasis in papillary thyroid microcarcinoma. Otolaryngol Head Neck Surg. 2012; 147:15–9.
Article8. Kwak JY, Kim EK, Kim MJ, Son EJ, Chung WY, Park CS, et al. Papillary microcarcinoma of the thyroid: predicting factors of lateral neck node metastasis. Ann Surg Oncol. 2009; 16:1348–55.
Article9. Jeon MJ, Kim WG, Choi YM, Kwon H, Lee YM, Sung TY, et al. Features predictive of distant metastasis in papillary thyroid microcarcinomas. Thyroid. 2016; 26:161–8.
Article10. Jeon MJ, Chung MS, Kwon H, Kim M, Park S, Baek JH, et al. Features of papillary thyroid microcarcinoma associated with lateral cervical lymph node metastasis. Clin Endocrinol (Oxf). 2017; 86:845–51.
Article11. Ghossein R, Ganly I, Biagini A, Robenshtok E, Rivera M, Tuttle RM. Prognostic factors in papillary microcarcinoma with emphasis on histologic subtyping: a clinicopathologic study of 148 cases. Thyroid. 2014; 24:245–53.
Article12. Kim K, Zheng X, Kim JK, Lee CR, Kang SW, Lee J, et al. The contributing factors for lateral neck lymph node metastasis in papillary thyroid microcarcinoma (PTMC). Endocrine. 2020; 69:149–56.
Article13. Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014; 159:676–90.14. Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012; 2:401–4.
Article15. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014; 15:550.
Article16. Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009; 4:44–57.
Article17. Newman AM, Steen CB, Liu CL, Gentles AJ, Chaudhuri AA, Scherer F, et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat Biotechnol. 2019; 37:773–82.
Article18. Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015; 12:453–7.
Article19. Cheng S, Li Z, Gao R, Xing B, Gao Y, Yang Y, et al. A pancancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell. 2021; 184:792–809.
Article20. Hanzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics. 2013; 14:7.
Article21. Jung KY, Cho SW, Kim YA, Kim D, Oh BC, Park DJ, et al. Cancers with higher density of tumor-associated macrophages were associated with poor survival rates. J Pathol Transl Med. 2015; 49:318–24.
Article22. Ruan J, Chen Z, Chen S, Xu Z, Wen L, Mao Z, et al. Lateral lymph node metastasis in papillary thyroid microcarcinoma: a study of 5241 follow-up patients. Endocrine. 2024; 83:414–21.
Article23. Lin DZ, Qu N, Shi RL, Lu ZW, Ji QH, Wu WL. Risk prediction and clinical model building for lymph node metastasis in papillary thyroid microcarcinoma. Onco Targets Ther. 2016; 9:5307–16.24. Liu Z, Lei J, Liu Y, Fan Y, Wang X, Lu X. Preoperative predictors of lateral neck lymph node metastasis in papillary thyroid microcarcinoma. Medicine (Baltimore). 2017; 96:e6240.
Article25. Attia AS, Hussein M, Issa PP, Elnahla A, Farhoud A, Magazine BM, et al. Association of BRAFV600E mutation with the aggressive behavior of papillary thyroid microcarcinoma: a meta-analysis of 33 studies. Int J Mol Sci. 2022; 23:15626.
Article26. Perera D, Ghossein R, Camacho N, Senbabaoglu Y, Seshan V, Li J, et al. Genomic and transcriptomic characterization of papillary microcarcinomas with lateral neck lymph node metastases. J Clin Endocrinol Metab. 2019; 104:4889–99.
Article27. Jeon MJ, Chun SM, Lee JY, Choi KW, Kim D, Kim TY, et al. Mutational profile of papillary thyroid microcarcinoma with extensive lymph node metastasis. Endocrine. 2019; 64:130–8.
Article28. Song YS, Kang BH, Lee S, Yoo SK, Choi YS, Park J, et al. Genomic and transcriptomic characteristics according to size of papillary thyroid microcarcinoma. Cancers (Basel). 2020; 12:1345.
Article29. Huynh KT, Hoon DS. Epigenetics of regional lymph node metastasis in solid tumors. Clin Exp Metastasis. 2012; 29:747–56.
Article30. Lin RX, Yang SL, Jia Y, Wu JC, Xu Z, Zhang H. Epigenetic regulation of papillary thyroid carcinoma by long non-coding RNAs. Semin Cancer Biol. 2022; 83:253–60.
Article31. Zhao F, Zhu S, Fang J, Dong H, Zhu C. Correlation of DNA methylation and lymph node metastasis in papillary thyroid carcinoma. Head Neck. 2023; 45:1654–62.32. Kim S, Cho SW, Min HS, Kim KM, Yeom GJ, Kim EY, et al. The expression of tumor-associated macrophages in papillary thyroid carcinoma. Endocrinol Metab (Seoul). 2013; 28:192–8.
Article33. Qing W, Fang WY, Ye L, Shen LY, Zhang XF, Fei XC, et al. Density of tumor-associated macrophages correlates with lymph node metastasis in papillary thyroid carcinoma. Thyroid. 2012; 22:905–10.
Article34. Pu W, Shi X, Yu P, Zhang M, Liu Z, Tan L, et al. Single-cell transcriptomic analysis of the tumor ecosystems underlying initiation and progression of papillary thyroid carcinoma. Nat Commun. 2021; 12:6058.
Article35. Lu L, Wang JR, Henderson YC, Bai S, Yang J, Hu M, et al. Anaplastic transformation in thyroid cancer revealed by single-cell transcriptomics. J Clin Invest. 2023; 133:e169653.
Article36. Kabasawa T, Ohe R, Aung NY, Urano Y, Kitaoka T, Tamazawa N, et al. Potential role of M2 TAMs around lymphatic vessels during lymphatic invasion in papillary thyroid carcinoma. Sci Rep. 2021; 11:1150.
Article37. Skaugen JM, Taneja C, Liu JB, Wald AI, Nikitski AV, Chiosea SI, et al. Performance of a multigene genomic classifier in thyroid nodules with suspicious for malignancy cytology. Thyroid. 2022; 32:1500–8.
Article38. Liu JB, Ramonell KM, Carty SE, McCoy KL, Schaitkin BM, Karslioglu-French E, et al. Association of comprehensive thyroid cancer molecular profiling with tumor phenotype and cancer-specific outcomes. Surgery. 2023; 173:252–9.
Article39. Liu JB, Baugh KA, Ramonell KM, McCoy KL, KarsliogluFrench E, Morariu EM, et al. Molecular testing predicts incomplete response to initial therapy in differentiated thyroid carcinoma without lateral neck or distant metastasis at presentation: retrospective cohort study. Thyroid. 2023; 33:705–14.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Cystic Lymph Node Metastasis from Thyroid Papillary Microcarcinoma
- Ultrasonographic Features of Metastatic Lymph Nodes in Papillary Thyroid Microcarcinomas and Macrocarcinomas
- Central Neck Lymph Node Metastasis from Papillary Thyroid Cancers
- Clinically Related Factors and Features of Central Compartment Neck Lymph Nodes in Thyroid Micropapillary Carcinoma
- A Case of Thyroid Papillary Microcarcinoma with Submental Node Metastasis