J Korean Med Sci.
2024 Apr;39(13):e104. 10.3346/jkms.2024.39.e104.
Application of the Hollow-Fiber Infection Model to Personalized Precision Dosing of Isoniazid in a Clinical Setting
- Affiliations
-
- 1Center for Personalized Precision Medicine of Tuberculosis, Inje University College of Medicine, Busan, Korea
- 2Department of Pharmacology and PharmacoGenomics Research Center, Inje University College of Medicine, Busan, Korea
- 3Department of Clinical Pharmacology, Inje University Busan Paik Hospital, Busan, Korea
- KMID: 2554576
- DOI: http://doi.org/10.3346/jkms.2024.39.e104
Abstract
- Background
The hollow-fiber infection model (HFIM) is a valuable tool for evaluating pharmacokinetics/pharmacodynamics relationships and determining the optimal antibiotic dose in monotherapy or combination therapy, but the application for personalized precision medicine in tuberculosis treatment remains limited. This study aimed to evaluate the efficacy of adjusted antibiotic doses for a tuberculosis patient using HFIM.
Methods
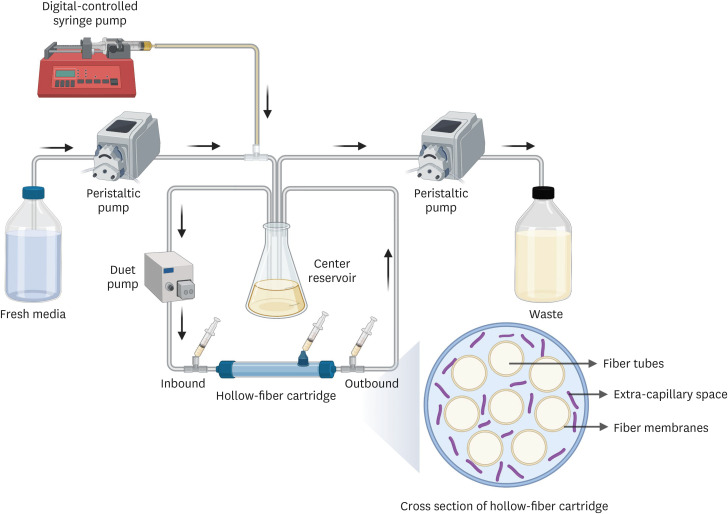
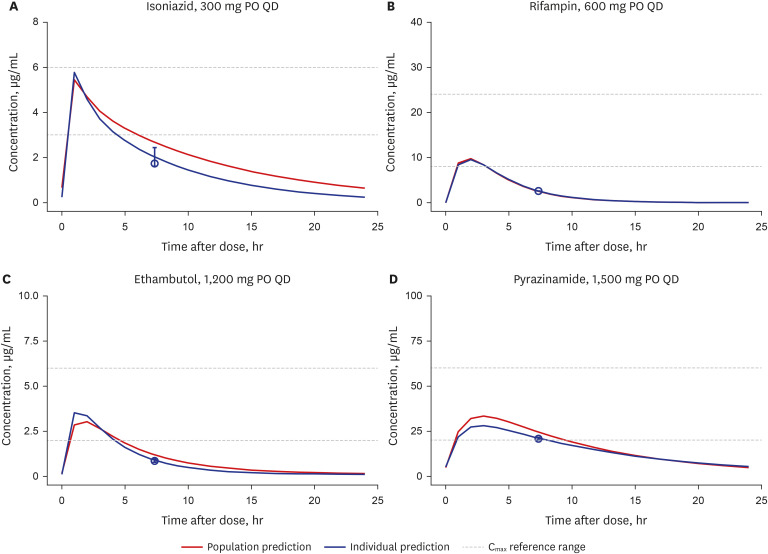
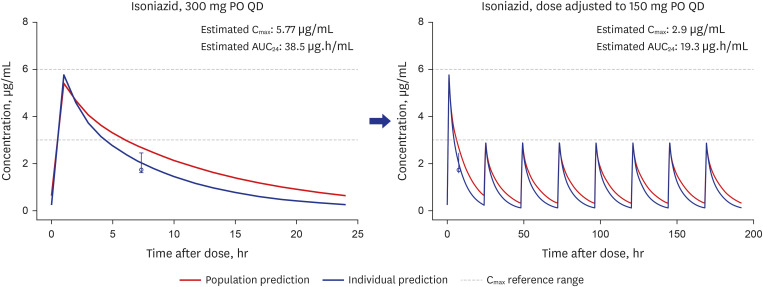
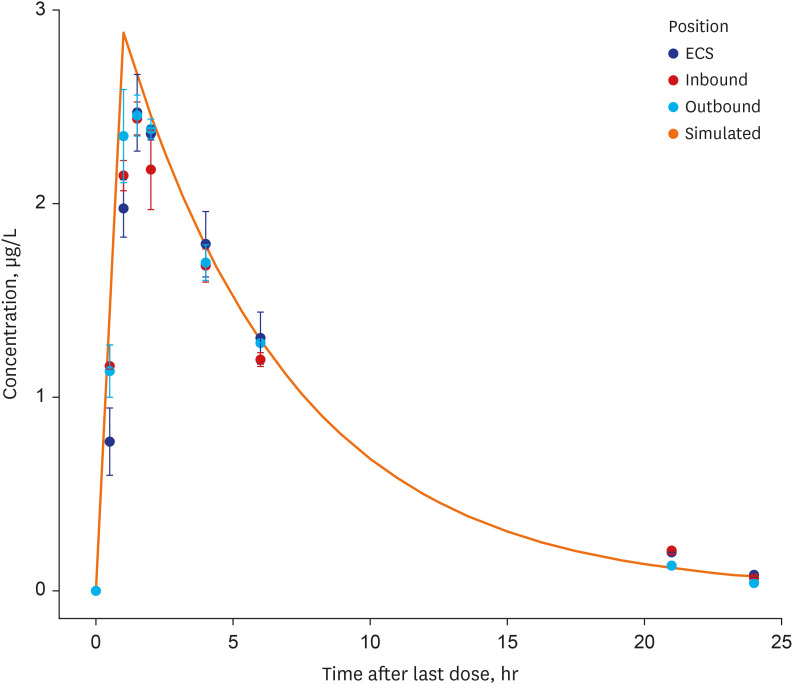
Model-based Bayesian forecasting was utilized to assess the proposed reduction of the isoniazid dose from 300 mg daily to 150 mg daily in a patient with an ultra-slowacetylation phenotype. The efficacy of the adjusted 150-mg dose was evaluated in a timeto-kill assay performed using the bacterial isolate Mycobacterium tuberculosis (Mtb) H37Ra in a HFIM that mimicked the individual pharmacokinetic profile of the patient.
Results
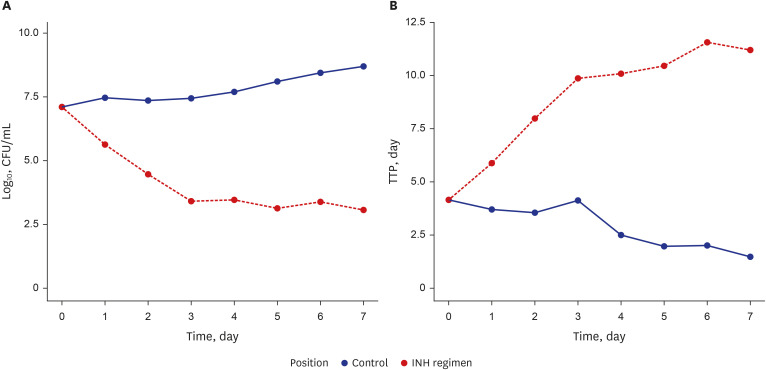
The isoniazid concentration observed in the HFIM adequately reflected the target drug exposures simulated by the model. After 7 days of repeated dose administration, isoniazid killed 4 log 10 Mtb CFU/mL in the treatment arm, while the control arm without isoniazid increased 1.6 log 10 CFU/mL.
Conclusion
Our results provide an example of the utility of the HFIM for predicting the efficacy of specific recommended doses of anti-tuberculosis drugs in real clinical setting.
Keyword
Figure
Reference
-
1. World Health Organization. Global Tuberculosis Report 2021. Geneva, Switzerland: World Health Organization;2021.2. Kim JH, Yim JJ. Achievements in and challenges of tuberculosis control in South Korea. Emerg Infect Dis. 2015; 21(11):1913–1920. PMID: 26485188.3. World Health Organization. Treatment of Tuberculosis: Guidelines. 4th ed. Geneva, Switzerland: World Health Organization;2010.4. Preziosi P. Isoniazid: metabolic aspects and toxicological correlates. Curr Drug Metab. 2007; 8(8):839–851. PMID: 18220565.5. Sundell J, Bienvenu E, Janzén D, Birgersson S, Äbelö A, Ashton M. Model-based assessment of variability in isoniazid pharmacokinetics and metabolism in patients co-infected with tuberculosis and HIV: implications for a novel dosing strategy. Clin Pharmacol Ther. 2020; 108(1):73–80. PMID: 32017035.6. Klein DJ, Boukouvala S, McDonagh EM, Shuldiner SR, Laurieri N, Thorn CF, et al. PharmGKB summary: isoniazid pathway, pharmacokinetics. Pharmacogenet Genomics. 2016; 26(9):436–444. PMID: 27232112.7. Parkin DP, Vandenplas S, Botha FJ, Vandenplas ML, Seifart HI, van Helden PD, et al. Trimodality of isoniazid elimination: phenotype and genotype in patients with tuberculosis. Am J Respir Crit Care Med. 1997; 155(5):1717–1722. PMID: 9154882.8. Peloquin CA, Jaresko GS, Yong CL, Keung AC, Bulpitt AE, Jelliffe RW. Population pharmacokinetic modeling of isoniazid, rifampin, and pyrazinamide. Antimicrob Agents Chemother. 1997; 41(12):2670–2679. PMID: 9420037.9. Evans DA, Manley KA, McKUSICK VA. Genetic control of isoniazid metabolism in man. BMJ. 1960; 2(5197):485–491. PMID: 13820968.10. Azuma J, Ohno M, Kubota R, Yokota S, Nagai T, Tsuyuguchi K, et al. NAT2 genotype guided regimen reduces isoniazid-induced liver injury and early treatment failure in the 6-month four-drug standard treatment of tuberculosis: a randomized controlled trial for pharmacogenetics-based therapy. Eur J Clin Pharmacol. 2013; 69(5):1091–1101. PMID: 23150149.11. Chung SJ, Byeon SJ, Choi JH. Analysis of adverse drug reactions to first-line anti-tuberculosis drugs using the Korea adverse event reporting system. J Korean Med Sci. 2022; 37(16):e128. PMID: 35470602.12. Badrinath M, John S. Isoniazid Toxicity. Treasure Island, FL, USA: StatPearls Publishing;2023.13. Gumbo T, Pasipanodya JG, Romero K, Hanna D, Nuermberger E. Forecasting accuracy of the hollow fiber model of tuberculosis for clinical therapeutic outcomes. Clin Infect Dis. 2015; 61(Suppl 1):S25–S31. PMID: 26224769.14. Gumbo T, Louie A, Liu W, Ambrose PG, Bhavnani SM, Brown D, et al. Isoniazid’s bactericidal activity ceases because of the emergence of resistance, not depletion of Mycobacterium tuberculosis in the log phase of growth. J Infect Dis. 2007; 195(2):194–201. PMID: 17191164.15. Gumbo T, Louie A, Liu W, Brown D, Ambrose PG, Bhavnani SM, et al. Isoniazid bactericidal activity and resistance emergence: integrating pharmacodynamics and pharmacogenomics to predict efficacy in different ethnic populations. Antimicrob Agents Chemother. 2007; 51(7):2329–2336. PMID: 17438043.16. Cho YS, Jang TW, Kim HJ, Oh JY, Lee HK, Park HK, et al. Isoniazid population pharmacokinetics and dose recommendation for Korean patients with tuberculosis based on target attainment analysis. J Clin Pharmacol. 2021; 61(12):1567–1578. PMID: 34157153.17. Kim HJ, Seo KA, Kim HM, Jeong ES, Ghim JL, Lee SH, et al. Simple and accurate quantitative analysis of 20 anti-tuberculosis drugs in human plasma using liquid chromatography-electrospray ionization-tandem mass spectrometry. J Pharm Biomed Anal. 2015; 102:9–16. PMID: 25218029.18. Tam VH, Schilling AN, Vo G, Kabbara S, Kwa AL, Wiederhold NP, et al. Pharmacodynamics of polymyxin B against Pseudomonas aeruginosa . Antimicrob Agents Chemother. 2005; 49(9):3624–3630. PMID: 16127031.19. Gumbo T, Louie A, Deziel MR, Parsons LM, Salfinger M, Drusano GL. Selection of a moxifloxacin dose that suppresses drug resistance in Mycobacterium tuberculosis, by use of an in vitro pharmacodynamic infection model and mathematical modeling. J Infect Dis. 2004; 190(9):1642–1651. PMID: 15478070.20. Bowness R, Boeree MJ, Aarnoutse R, Dawson R, Diacon A, Mangu C, et al. The relationship between Mycobacterium tuberculosis MGIT time to positivity and cfu in sputum samples demonstrates changing bacterial phenotypes potentially reflecting the impact of chemotherapy on critical sub-populations. J Antimicrob Chemother. 2015; 70(2):448–455. PMID: 25344806.21. Erwin ER, Addison AP, John SF, Olaleye OA, Rosell RC. Pharmacokinetics of isoniazid: the good, the bad, and the alternatives. Tuberculosis (Edinb). 2019; 116S:S66–S70. PMID: 31076322.22. Donald PR, Sirgel FA, Venter A, Parkin DP, Seifart HI, van de Wal BW, et al. The influence of human N-acetyltransferase genotype on the early bactericidal activity of isoniazid. Clin Infect Dis. 2004; 39(10):1425–1430. PMID: 15546075.23. Wang P, Pradhan K, Zhong XB, Ma X. Isoniazid metabolism and hepatotoxicity. Acta Pharm Sin B. 2016; 6(5):384–392. PMID: 27709007.24. Cavaleri M, Manolis E. Hollow fiber system model for tuberculosis: the european medicines agency experience. Clin Infect Dis. 2015; 61(Suppl 1):S1–S4. PMID: 26224766.25. Gumbo T, Srivastava S, Deshpande D, Pasipanodya JG, Berg A, Romero K, et al. Hollow-fibre system model of tuberculosis reproducibility and performance specifications for best practice in drug and combination therapy development. J Antimicrob Chemother. 2023; 78(4):953–964. PMID: 36794692.26. Drusano GL, Myrick J, Maynard M, Nole J, Duncanson B, Brown D, et al. Linezolid kills acid-phase and nonreplicative-persister-phase Mycobacterium tuberculosis in a hollow-fiber infection model. Antimicrob Agents Chemother. 2018; 62(8):e00221-18. PMID: 29866864.27. Louie A, Duncanson B, Myrick J, Maynard M, Nole J, Brown D, et al. Activity of moxifloxacin against Mycobacterium tuberculosis in acid phase and nonreplicative-persister phenotype phase in a hollow-fiber infection model. Antimicrob Agents Chemother. 2018; 62(12):e01470-18. PMID: 30249693.28. Srivastava S, Deshpande D, Magombedze G, Gumbo T. Efficacy versus hepatotoxicity of high-dose rifampin, pyrazinamide, and moxifloxacin to shorten tuberculosis therapy duration: there is still fight in the old warriors yet! Clin Infect Dis. 2018; 67(Suppl 3):S359–S364. PMID: 30496465.29. Gumbo T, Sherman CM, Deshpande D, Alffenaar JW, Srivastava S. Mycobacterium tuberculosis sterilizing activity of faropenem, pyrazinamide and linezolid combination and failure to shorten the therapy duration. Int J Infect Dis. 2021; 104:680–684. PMID: 33556616.30. Srivastava S, Deshpande D, Magombedze G, van Zyl J, Cirrincione K, Martin K, et al. Duration of pretomanid/moxifloxacin/pyrazinamide therapy compared with standard therapy based on time-to-extinction mathematics. J Antimicrob Chemother. 2020; 75(2):392–399. PMID: 31713607.31. Magombedze G, Pasipanodya JG, Srivastava S, Deshpande D, Visser ME, Chigutsa E, et al. Transformation morphisms and time-to-extinction analysis that map therapy duration from preclinical models to patients with tuberculosis: translating from apples to oranges. Clin Infect Dis. 2018; 67(Suppl 3):S349–S358. PMID: 30496464.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ultra-rare Disease and Genomics-Driven Precision Medicine
- Correlative Effect between in vivo Hollow Fiber Assay and Xenografts Assay in Drug Screening
- In vivo Hollow Fiber Assay for Anticancer Drugs' Responsiveness in a Bladder Cancer Model
- Building and analyzing machine learning-based warfarin dose prediction models using scikit-learn
- Personalized Medicine in Cardiovascular Diseases