Cancer Res Treat.
2024 Apr;56(2):602-615. 10.4143/crt.2023.726.
A Single-Arm Phase II Study of Nab-Paclitaxel Plus Gemcitabine and Cisplatin for Locally Advanced or Metastatic Biliary Tract Cancer
- Affiliations
-
- 1Department of Biotherapy, Cancer Center, West China Hospital, Sichuan University, Sichuan, China
- 2Thoracic Oncology Ward, Cancer Center, West China Hospital, Sichuan University, Sichuan, China
- 3Department of Abdominal Oncology, Cancer Center, West China Hospital, Sichuan University, Sichuan, China
- 4Department of Gastric Cancer Center, Division of Medical Oncology, Cancer Center, West China Hospital, Sichuan University, Sichuan, China
- KMID: 2554349
- DOI: http://doi.org/10.4143/crt.2023.726
Abstract
- Purpose
Patients with advanced biliary tract cancer (BTC) have a poor survival. We aim to evaluate the efficacy and safety of nab-paclitaxel plus gemcitabine and cisplatin regimen in Chinese advanced BTC patients.
Materials and Methods
Eligible patients with locally advanced or metastatic BTC administrated intravenous 100 mg/m2 nab-paclitaxel, 800 mg/m2 gemcitabine, and 25 mg/m2 cisplatin every 3 weeks. The primary endpoint was progression-free survival (PFS). The secondary endpoints included overall survival (OS) and adverse events, while exploratory endpoint was the association of biomarkers with efficacy.
Results
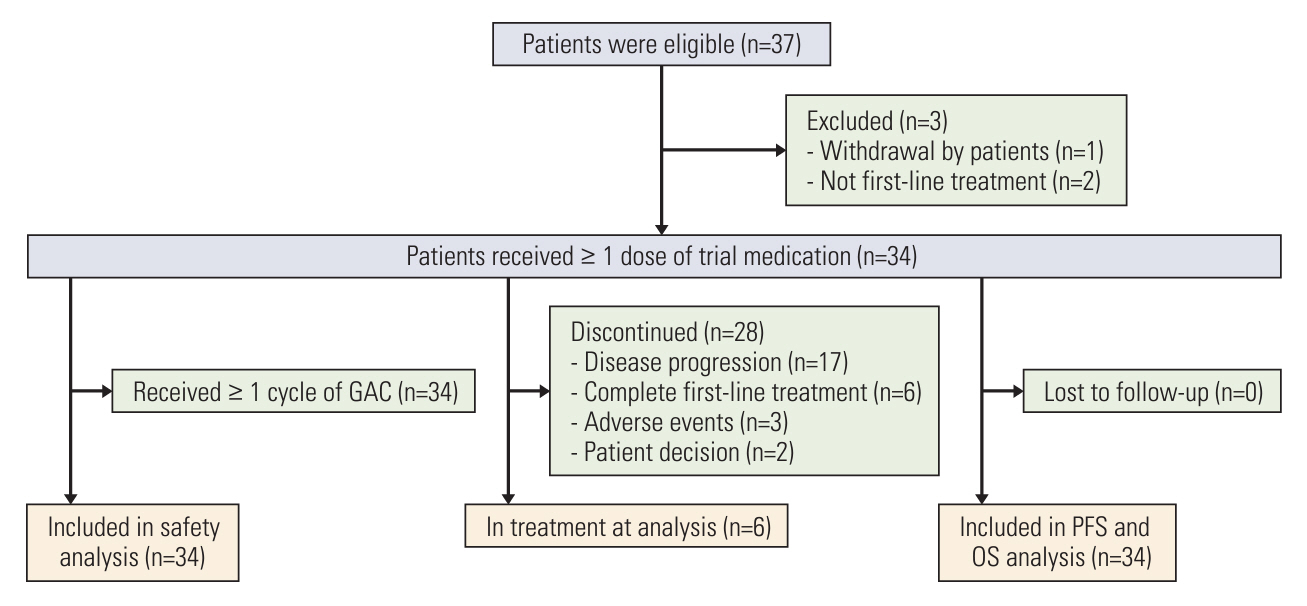
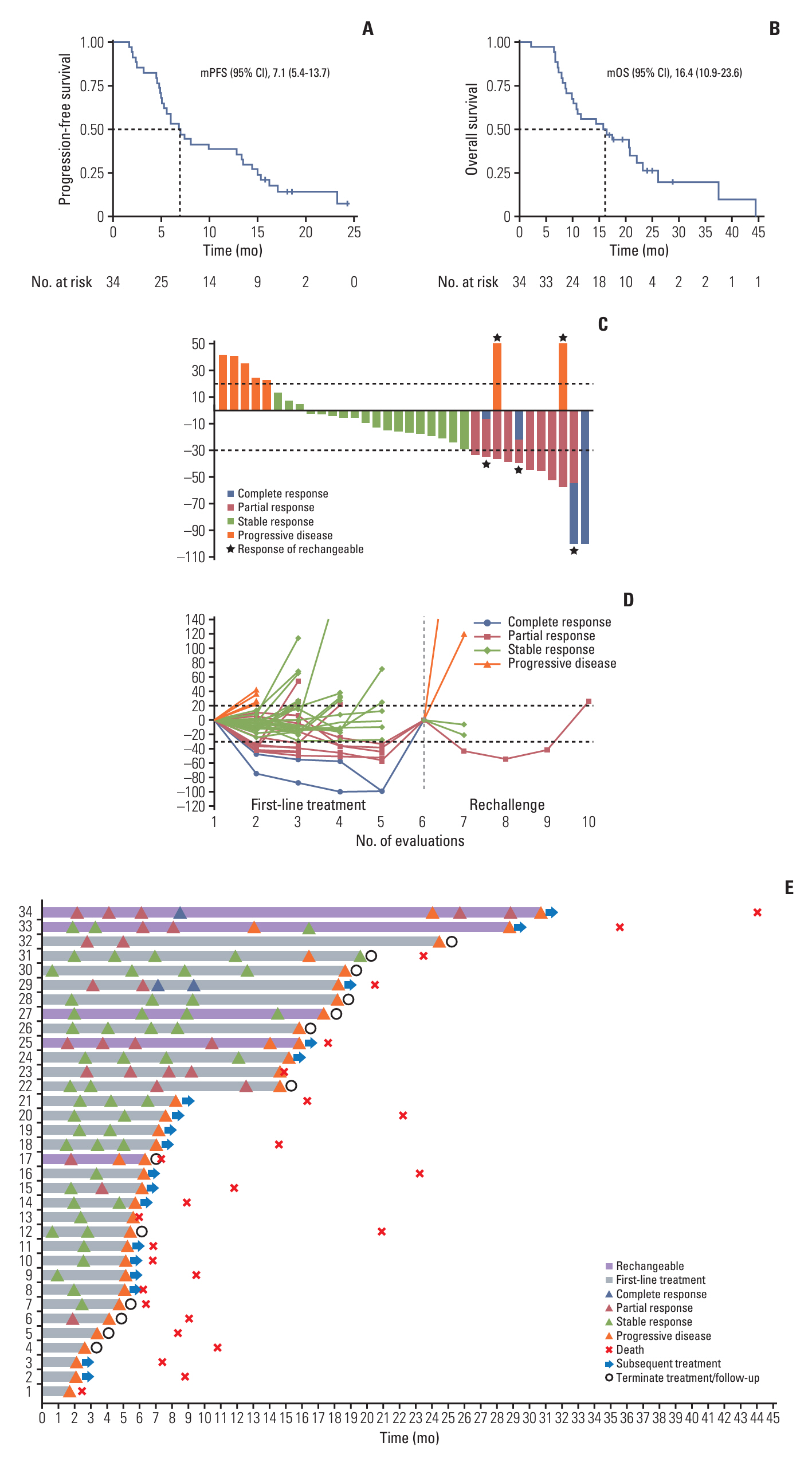
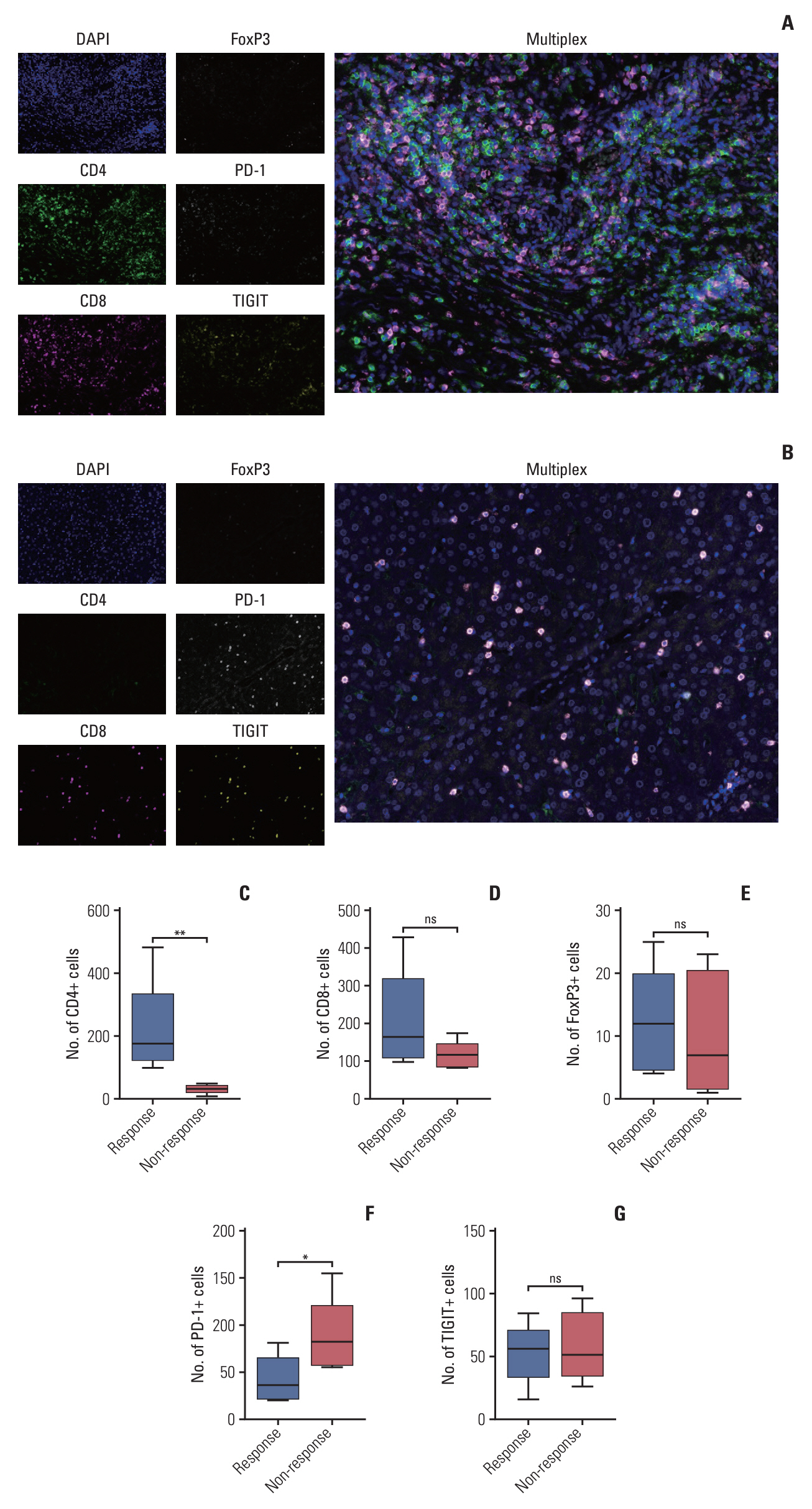
After the median follow-up of 25.0 months, the median PFS and OS of 34 enrolled patients were 7.1 months (95% confidence interval [CI], 5.4 to 13.7) and 16.4 months (95% CI, 10.9 to 23.6), respectively. The most common treatment-related adverse events at ≥ 3 grade were neutropenia (26.5%) and leukopenia (26.5%). Survival analyses demonstrated that carcinoembryonic antigen (CEA) levels could monitor patients’ survival outcomes. A significant increase in the number of infiltrating CD4+ cells (p=0.008) and a decrease in programmed death-1–positive (PD-1+) cells (p=0.032) were observed in the response patients.
Conclusion
In advanced BTC patients, nab-paclitaxel plus gemcitabine and cisplatin regimen showed therapeutic potential. Potential prognostic factors of CEA levels, number of CD4+ cells and PD-1+ cells may help us maximize the efficacy benefit.
Figure
Reference
-
References
1. Valle JW, Kelley RK, Nervi B, Oh DY, Zhu AX. Biliary tract cancer. Lancet. 2021; 397:428–44.
Article2. Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016; 13:261–80.
Article3. Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-year trends in cholangiocarcinoma incidence in the U.S.: intrahepatic disease on the rise. Oncologist. 2016; 21:594–9.
Article4. Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014; 60:1268–89.
Article5. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010; 362:1273–81.
Article6. Komaya K, Ebata T, Shirai K, Ohira S, Morofuji N, Akutagawa A, et al. Recurrence after resection with curative intent for distal cholangiocarcinoma. Br J Surg. 2017; 104:426–33.
Article7. Komaya K, Ebata T, Yokoyama Y, Igami T, Sugawara G, Mizuno T, et al. Recurrence after curative-intent resection of perihilar cholangiocarcinoma: analysis of a large cohort with a close postoperative follow-up approach. Surgery. 2018; 163:732–8.
Article8. Valle JW, Furuse J, Jitlal M, Beare S, Mizuno N, Wasan H, et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol. 2014; 25:391–8.
Article9. Kim BJ, Hyung J, Yoo C, Kim KP, Park SJ, Lee SS, et al. Prognostic factors in patients with advanced biliary tract cancer treated with first-line gemcitabine plus cisplatin: retrospective analysis of 740 patients. Cancer Chemother Pharmacol. 2017; 80:209–15.
Article10. Shroff RT, Javle MM, Xiao L, Kaseb AO, Varadhachary GR, Wolff RA, et al. Gemcitabine, cisplatin, and nab-paclitaxel for the treatment of advanced biliary tract cancers: a phase 2 clinical trial. JAMA Oncol. 2019; 5:824–30.
Article11. Jung K, Park J, Jung JH, Lee JC, Kim J, Hwang JH. Real-world outcomes of gemcitabine, cisplatin, and nab-paclitaxel chemotherapy regimen for advanced biliary tract cancer: a propensity score-matched analysis. Gut Liver. 2022; 16:798–805.
Article12. Ouyang G, Liu Q, Wu Y, Liu Z, Lu W, Li S, et al. The global, regional, and national burden of gallbladder and biliary tract cancer and its attributable risk factors in 195 countries and territories, 1990 to 2017: a systematic analysis for the Global Burden of Disease Study 2017. Cancer. 2021; 127:2238–50.
Article13. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–49.
Article14. Sahai V, Catalano PJ, Zalupski MM, Lubner SJ, Menge MR, Nimeiri HS, et al. Nab-paclitaxel and gemcitabine as first-line treatment of advanced or metastatic cholangiocarcinoma: a phase 2 clinical trial. JAMA Oncol. 2018; 4:1707–12.
Article15. You MS, Ryu JK, Choi YH, Choi JH, Huh G, Paik WH, et al. Therapeutic outcomes and prognostic factors in unresectable gallbladder cancer treated with gemcitabine plus cisplatin. BMC Cancer. 2019; 19:10.
Article16. Lamarca A, Ross P, Wasan HS, Hubner RA, McNamara MG, Lopes A, et al. Advanced intrahepatic cholangiocarcinoma: post hoc analysis of the ABC-01, -02, and -03 clinical trials. J Natl Cancer Inst. 2020; 112:200–10.
Article17. Kang J, Lee SH, Son JH, Lee JW, Choi YH, Choi JH, et al. Body mass index and weight change during initial period of chemotherapy affect survival outcome in advanced biliary tract cancer patients. PLoS One. 2018; 13:e0195118.
Article18. Bouillanne O, Dupont-Belmont C, Hay P, Hamon-Vilcot B, Cynober L, Aussel C. Fat mass protects hospitalized elderly persons against morbidity and mortality. Am J Clin Nutr. 2009; 90:505–10.
Article19. Gonzalez MC, Pastore CA, Orlandi SP, Heymsfield SB. Obesity paradox in cancer: new insights provided by body composition. Am J Clin Nutr. 2014; 99:999–1005.
Article20. Meza-Junco J, Montano-Loza AJ, Baracos VE, Prado CM, Bain VG, Beaumont C, et al. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J Clin Gastroenterol. 2013; 47:861–70.
Article21. Sabel MS, Lee J, Cai S, Englesbe MJ, Holcombe S, Wang S. Sarcopenia as a prognostic factor among patients with stage III melanoma. Ann Surg Oncol. 2011; 18:3579–85.
Article22. Peixoto RD, Renouf D, Lim H. A population based analysis of prognostic factors in advanced biliary tract cancer. J Gastrointest Oncol. 2014; 5:428–32.23. Wang YF, Feng FL, Zhao XH, Ye ZX, Zeng HP, Li Z, et al. Combined detection tumor markers for diagnosis and prognosis of gallbladder cancer. World J Gastroenterol. 2014; 20:4085–92.
Article24. Park HS, Park JS, Chun YJ, Roh YH, Moon J, Chon HJ, et al. Prognostic factors and scoring model for survival in metastatic biliary tract cancer. Cancer Res Treat. 2017; 49:1127–39.
Article25. Kitano Y, Okabe H, Yamashita YI, Nakagawa S, Saito Y, Umezaki N, et al. Tumour-infiltrating inflammatory and immune cells in patients with extrahepatic cholangiocarcinoma. Br J Cancer. 2018; 118:171–80.
Article26. Goeppert B, Frauenschuh L, Zucknick M, Stenzinger A, Andrulis M, Klauschen F, et al. Prognostic impact of tumour-infiltrating immune cells on biliary tract cancer. Br J Cancer. 2013; 109:2665–74.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Novel Palliative Chemotherapy for Cholangiocarcinoma
- Real-World Outcomes of Gemcitabine, Cisplatin, and Nab-Paclitaxel Chemotherapy Regimen for Advanced Biliary Tract Cancer: A Propensity Score-Matched Analysis
- Chemotherapy for Biliary Tract Cancer
- Phase II Study of Cisplatin, Ifosfamide . Paclitaxel (CIP) as Neoadjuvant Chemotherapy in Patients with Locally Advanced Cervical Carcinoma
- Chemotherapy and Targeted Therapy with Management of Related Complications in Pancreatic Cancer