Cancer Res Treat.
2024 Apr;56(2):531-537. 10.4143/crt.2023.996.
Implication of Pre- and Post-radiotherapy ctDNA Dynamics in Patients with Residual Triple-Negative Breast Cancer at Surgery after Neoadjuvant Chemotherapy: Findings from a Prospective Observational Study
- Affiliations
-
- 1Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Samsung Genome Institute, Samsung Medical Center, Seoul, Korea
- 3Department of Molecular Cell Biology, Sungkyunkwan University School of Medicine, Suwon, Korea
- KMID: 2554342
- DOI: http://doi.org/10.4143/crt.2023.996
Abstract
- Purpose
This study aims to determine the association between pre- and postoperative radiotherapy (PORT) circulating tumor DNA (ctDNA) dynamics and oncological outcomes in patients with residual triple-negative breast cancer who underwent surgery after neoadjuvant chemotherapy (NAC).
Materials and Methods
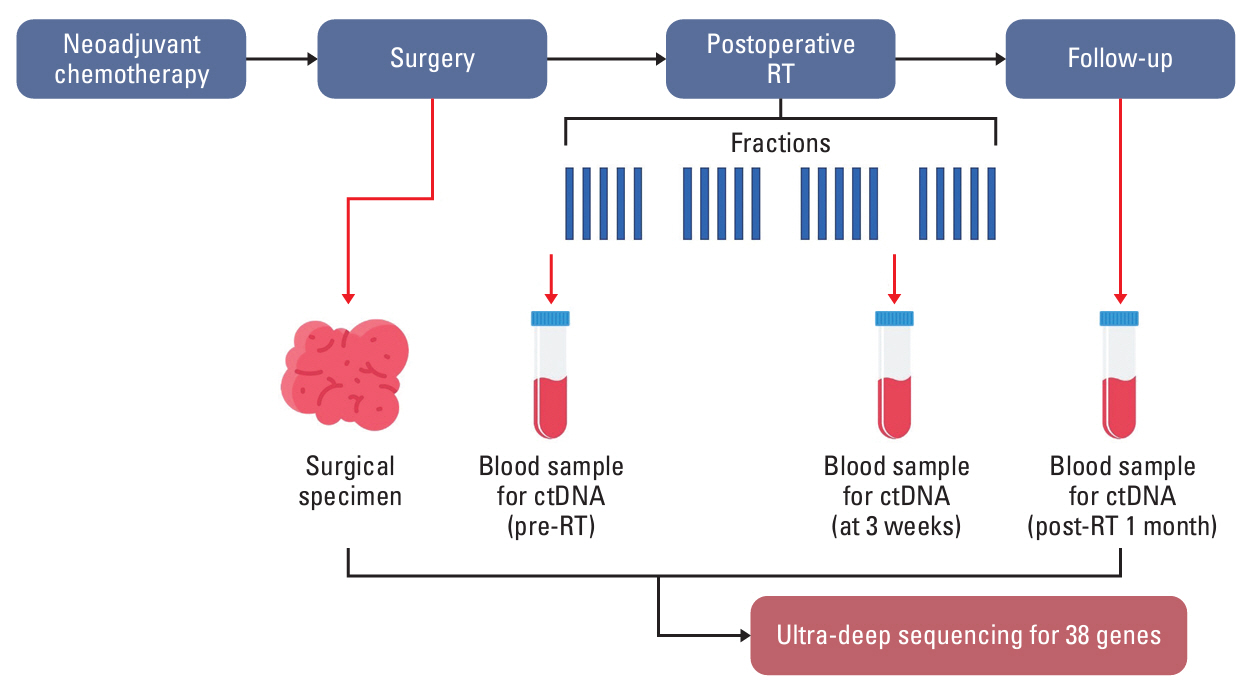
Between March 2019 and July 2020, 11 nonmetastatic patients with residual disease who underwent surgery after NAC were prospectively enrolled. In each patient, tumor specimens obtained during surgery and blood samples collected at three time points during PORT (T0: pre-PORT, T1: 3 weeks after PORT, T2: 1 month after PORT) were sequenced, targeting 38 cancer-related genes. Disease-free survival (DFS) was evaluated and the association between DFS and ctDNA dynamics was analyzed.
Results
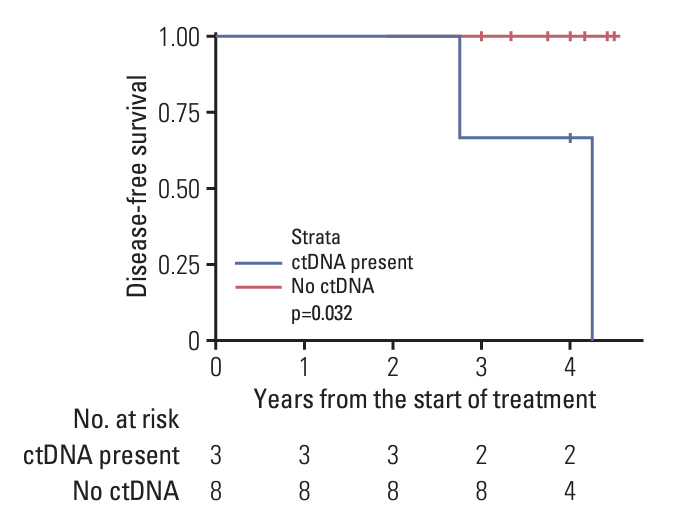
At T0, ctDNA was detected in three (27.2%) patients. The ctDNA dynamics were as follows: two showed a decreasing ctDNA variant allele frequency (VAF) and reached zero VAF at T2, while one patient exhibited an increasing VAF during PORT and maintained an elevated VAF at T2. After a median follow-up of 48 months, two patients experienced distant metastasis without any locoregional failures. All failures occurred in patients with ctDNA positivity at T0 and a decreased VAF after PORT. The 4-year DFS rates according to the T0 ctDNA status were 67% (positive ctDNA) and 100% (negative ctDNA) (p=0.032).
Conclusion
More than a quarter of the patients with residual disease after post-NAC surgery exhibited pre-PORT ctDNA positivity, and ctDNA positivity was associated with poor DFS. For patients with pre-PORT ctDNA positivity, the administration of a more effective systemic treatment should be considered.
Figure
Reference
-
References
1. Kang MJ, Won YJ, Lee JJ, Jung KW, Kim HJ, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2019. Cancer Res Treat. 2022; 54:330–44.
Article2. Blows FM, Driver KE, Schmidt MK, Broeks A, van Leeuwen FE, Wesseling J, et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010; 7:e1000279.
Article3. Ignatiadis M, Sledge GW, Jeffrey SS. Liquid biopsy enters the clinic: implementation issues and future challenges. Nat Rev Clin Oncol. 2021; 18:297–312.
Article4. Cescon DW, Bratman SV, Chan SM, Siu LL. Circulating tumor DNA and liquid biopsy in oncology. Nat Cancer. 2020; 1:276–90.
Article5. Korde LA, Somerfield MR, Carey LA, Crews JR, Denduluri N, Hwang ES, et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin Oncol. 2021; 39:1485–505.
Article6. Spring LM, Fell G, Arfe A, Sharma C, Greenup R, Reynolds KL, et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: a comprehensive meta-analysis. Clin Cancer Res. 2020; 26:2838–48.
Article7. Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES, Yokota I, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017; 376:2147–59.
Article8. Kim H, Kim YJ, Park D, Park WY, Choi DH, Park W, et al. Dynamics of circulating tumor DNA during postoperative radiotherapy in patients with residual triple-negative breast cancer following neoadjuvant chemotherapy: a prospective observational study. Breast Cancer Res Treat. 2021; 189:167–75.
Article9. Li S, Lai H, Liu J, Liu Y, Jin L, Li Y, et al. Circulating tumor DNA predicts the response and prognosis in patients with early breast cancer receiving neoadjuvant chemotherapy. JCO Precis Oncol. 2020; 4:244–57.
Article10. Cavallone L, Aguilar-Mahecha A, Lafleur J, Brousse S, Aldamry M, Roseshter T, et al. Prognostic and predictive value of circulating tumor DNA during neoadjuvant chemotherapy for triple negative breast cancer. Sci Rep. 2020; 10:14704.
Article11. Ortolan E, Appierto V, Silvestri M, Miceli R, Veneroni S, Folli S, et al. Blood-based genomics of triple-negative breast cancer progression in patients treated with neoadjuvant chemotherapy. ESMO Open. 2021; 6:100086.12. Magbanua MJ, Swigart LB, Wu HT, Hirst GL, Yau C, Wolf DM, et al. Circulating tumor DNA in neoadjuvant-treated breast cancer reflects response and survival. Ann Oncol. 2021; 32:229–39.
Article13. Cailleux F, Agostinetto E, Lambertini M, Rothe F, Wu HT, Balcioglu M, et al. Circulating tumor DNA after neoadjuvant chemotherapy in breast cancer is associated with disease relapse. JCO Precis Oncol. 2022; 6:e2200148.
Article14. Radovich M, Jiang G, Hancock BA, Chitambar C, Nanda R, Falkson C, et al. Association of circulating tumor DNA and circulating tumor cells after neoadjuvant chemotherapy with disease recurrence in patients with triple-negative breast cancer: preplanned secondary analysis of the BRE12-158 randomized clinical trial. JAMA Oncol. 2020; 6:1410–5.
Article15. Moser T, Waldispuehl-Geigl J, Belic J, Weber S, Zhou Q, Hasenleithner SO, et al. On-treatment measurements of circulating tumor DNA during FOLFOX therapy in patients with colorectal cancer. NPJ Precis Oncol. 2020; 4:30.
Article16. Song Y, Hu C, Xie Z, Wu L, Zhu Z, Rao C, et al. Circulating tumor DNA clearance predicts prognosis across treatment regimen in a large real-world longitudinally monitored advanced non-small cell lung cancer cohort. Transl Lung Cancer Res. 2020; 9:269–79.
Article17. Han K, Leung E, Barbera L, Barnes E, Croke J, Di Grappa MA, et al. Circulating human papillomavirus DNA as a biomarker of response in patients with locally advanced cervical cancer treated with definitive chemoradiation. JCO Precis Oncol. 2018; 2:1–8.
Article18. He SS, Wang Y, Bao Y, Cai XY, Yang XL, Chen DM, et al. Dynamic changes in plasma Epstein-Barr virus DNA load during treatment have prognostic value in nasopharyngeal carcinoma: a retrospective study. Cancer Med. 2018; 7:1110–7.
Article19. Jia R, Zhao CH, Li PS, Liu RR, Zhang Y, Chen HE, et al. Postradiation circulating tumor DNA as a prognostic factor in locally advanced esophageal squamous cell carcinoma. Oncol Lett. 2021; 21:68.
Article20. Tie J, Kinde I, Wang Y, Wong HL, Roebert J, Christie M, et al. Circulating tumor DNA as an early marker of therapeutic response in patients with metastatic colorectal cancer. Ann Oncol. 2015; 26:1715–22.
Article21. Kruger S, Heinemann V, Ross C, Diehl F, Nagel D, Ormanns S, et al. Repeated mutKRAS ctDNA measurements represent a novel and promising tool for early response prediction and therapy monitoring in advanced pancreatic cancer. Ann Oncol. 2018; 29:2348–55.
Article22. Bratman SV, Yang SY, Iafolla MA, Liu Z, Hansen AR, Bedard PL, et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat Cancer. 2020; 1:873–81.
Article23. McDonald KA, Kawaguchi T, Qi Q, Peng X, Asaoka M, Young J, et al. Tumor heterogeneity correlates with less immune response and worse survival in breast cancer patients. Ann Surg Oncol. 2019; 26:2191–9.
Article24. Ma F, Guan Y, Yi Z, Chang L, Li Q, Chen S, et al. Assessing tumor heterogeneity using ctDNA to predict and monitor therapeutic response in metastatic breast cancer. Int J Cancer. 2020; 146:1359–68.
Article25. Gao J, Wang H, Zang W, Li B, Rao G, Li L, et al. Circulating tumor DNA functions as an alternative for tissue to overcome tumor heterogeneity in advanced gastric cancer. Cancer Sci. 2017; 108:1881–7.26. Perdigones N, Murtaza M. Capturing tumor heterogeneity and clonal evolution in solid cancers using circulating tumor DNA analysis. Pharmacol Ther. 2017; 174:22–6.
Article27. Fridland S, Choi J, Nam M, Schellenberg SJ, Kim E, Lee G, et al. Assessing tumor heterogeneity: integrating tissue and circulating tumor DNA (ctDNA) analysis in the era of immunooncology: blood TMB is not the same as tissue TMB. J Immunother Cancer. 2021; 9:e002551.28. Turner NC, Kingston B, Kilburn LS, Kernaghan S, Wardley AM, Macpherson IR, et al. Circulating tumour DNA analysis to direct therapy in advanced breast cancer (plasmaMATCH): a multicentre, multicohort, phase 2a, platform trial. Lancet Oncol. 2020; 21:1296–308.
Article29. Kim A, Jang MH, Lee SJ, Bae YK. Mutations of the epidermal growth factor receptor gene in triple-negative breast cancer. J Breast Cancer. 2017; 20:150–9.
Article30. Schmid P, Cortes J, Pusztai L, McArthur H, Kummel S, Bergh J, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020; 382:810–21.
Article31. Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020; 396:1817–28.32. Turner NC, Swift C, Jenkins B, Kilburn L, Coakley M, Beaney M, et al. Results of the c-TRAK TN trial: a clinical trial utilising ctDNA mutation tracking to detect molecular residual disease and trigger intervention in patients with moderate- and high-risk early-stage triple-negative breast cancer. Ann Oncol. 2023; 34:200–11.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pathologic Findings of Residual Tumor according to the Response Rate after Neoadjuvant Chemotherapy for Breast Cancer
- Comment on “Histomorphological Factors Predicting the Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancerâ€
- Predictive and Prognostic Roles of Pathological Indicators for Patients with Breast Cancer on Neoadjuvant Chemotherapy
- Differences in prognosis by p53 expression after neoadjuvant chemotherapy in triple-negative breast cancer
- Bilateral Triple Negative Invasive Ductal Breast Carcinoma in a BRCA1 Mutation Carrier with Discrepant Pathologic Response to Neoadjuvant Chemotherapy