Cancer Res Treat.
2024 Apr;56(2):502-512. 10.4143/crt.2023.840.
Clinical Effect of Endosonography on Overall Survival in Patients with Radiological N1 Non–Small Cell Lung Cancer
- Affiliations
-
- 1Division of Pulmonary Medicine and Allergy, Department of Internal Medicine, Hanyang University College of Medicine, Seoul, Korea
- 2Division of Pulmonary and Critical Care Medicine, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 3Biomedical Statistics Center, Research Institute for Future Medicine, Samsung Medical Center, Seoul, Korea
- 4Department of Thoracic and Cardiovascular Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2554339
- DOI: http://doi.org/10.4143/crt.2023.840
Abstract
- Purpose
It is unclear whether performing endosonography first in non–small cell lung cancer (NSCLC) patients with radiological N1 (rN1) has any advantages over surgery without nodal staging. We aimed to compare surgery without endosonography to performing endosonography first in rN1 on the overall survival (OS) of patients with NSCLC.
Materials and Methods
This is a retrospective analysis of patients with rN1 NSCLC between 2013 and 2019. Patients were divided into ‘no endosonography’ and ‘endosonography first’ groups. We investigated the effect of nodal staging through endosonography on OS using propensity score matching (PSM) and multivariable Cox proportional hazard regression analysis.
Results
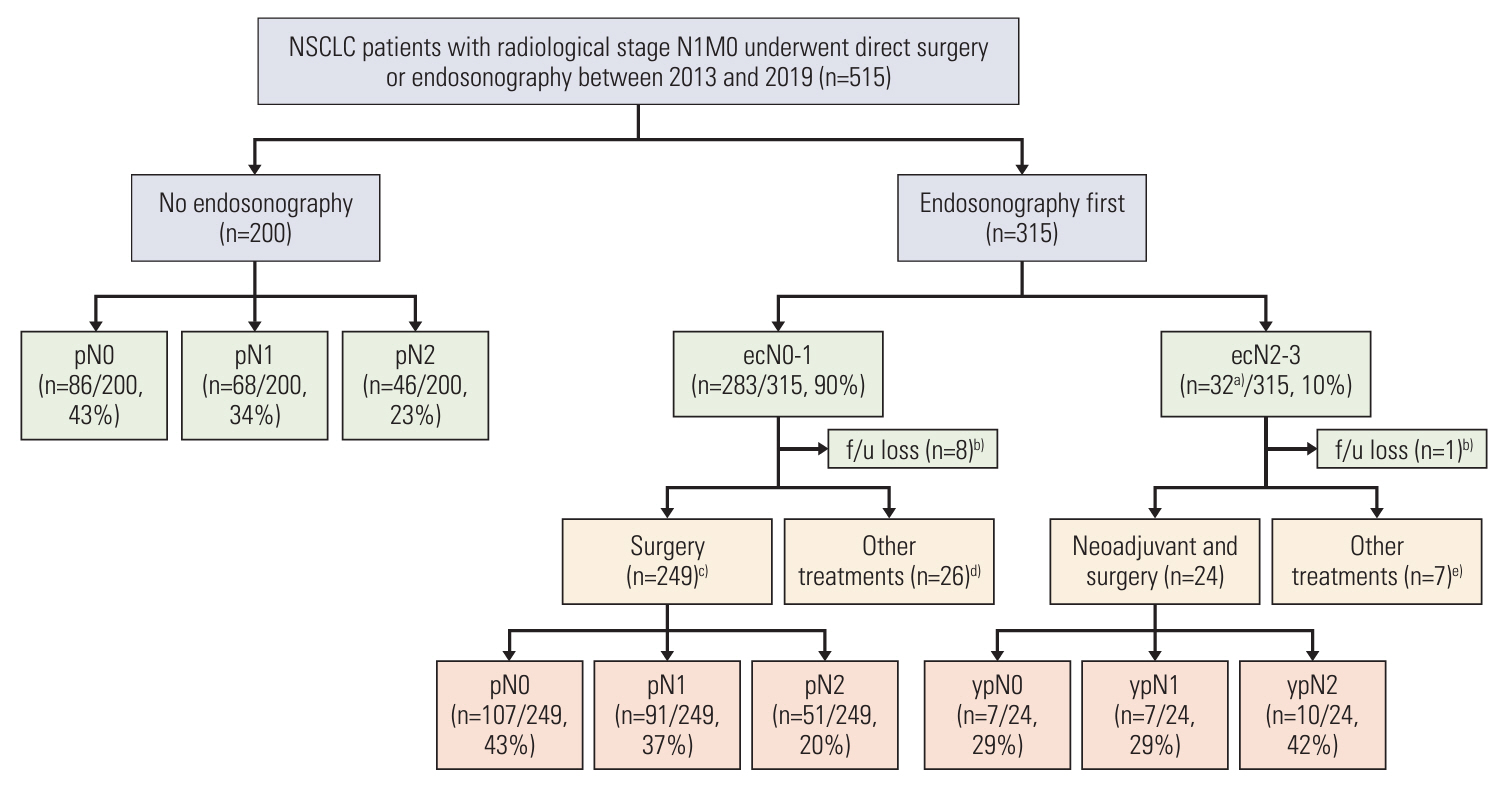
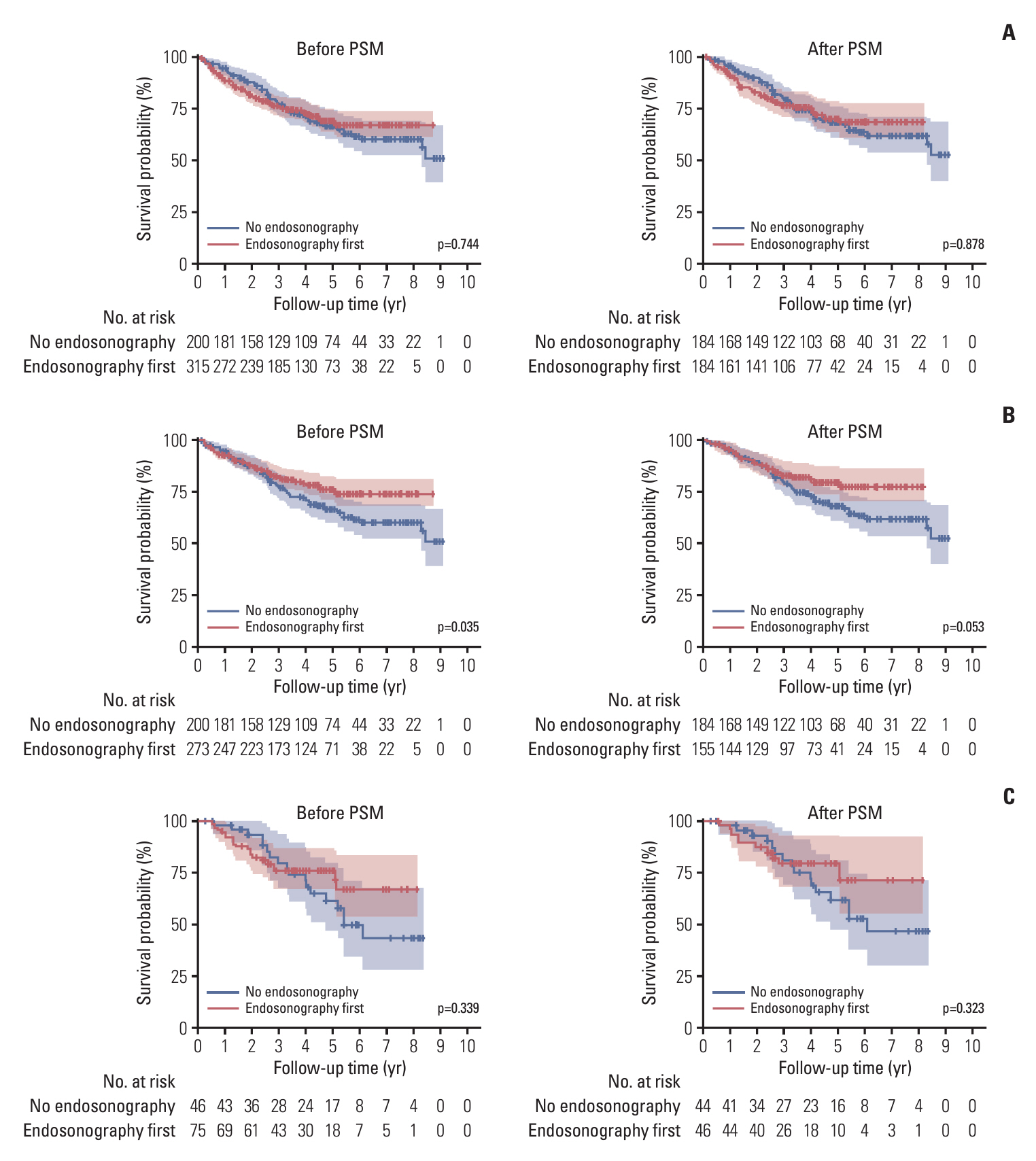
In the no endosonography group, pathologic N2 occurred in 23.0% of patients. In the endosonography first group, endosonographic N2 and N3 occurred in 8.6% and 1.6% of patients, respectively. Additionally, 51 patients were pathologic N2 among 249 patients who underwent surgery and mediastinal lymph node dissection (MLND) in endosonography first group. After PSM, the 5-year OSs were 68.1% and 70.6% in the no endosonography and endosonography first groups, respectively. However, the 5-year OS was 80.2% in the subgroup who underwent surgery and MLND of the endosonography first group. Moreover, in patients receiving surgical resection with MLND, the endosonography first group tended to have a better OS than the no endosonography group in adjusted analysis using various models.
Conclusion
In rN1 NSCLC, preoperative endosonography shows better OS than surgery without endosonography. For patients with rN1 NSCLC who are candidates for surgery, preoperative endosonography may help improve survival through patient selection.
Keyword
Figure
Reference
-
References
1. De Leyn P, Dooms C, Kuzdzal J, Lardinois D, Passlick B, Rami-Porta R, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg. 2014; 45:787–98.
Article2. Vilmann P, Clementsen PF, Colella S, Siemsen M, De Leyn P, Dumonceau JM, et al. Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). Endoscopy. 2015; 47:c1.
Article3. Yasufuku K, Chiyo M, Sekine Y, Chhajed PN, Shibuya K, Iizasa T, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest. 2004; 126:122–8.
Article4. Varela-Lema L, Fernandez-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: a systematic review. Eur Respir J. 2009; 33:1156–64.
Article5. Lee HS, Lee GK, Lee HS, Kim MS, Lee JM, Kim HY, et al. Realtime endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal staging of non-small cell lung cancer: how many aspirations per target lymph node station? Chest. 2008; 134:368–74.
Article6. Hishida T, Yoshida J, Nishimura M, Nishiwaki Y, Nagai K. Problems in the current diagnostic standards of clinical N1 non-small cell lung cancer. Thorax. 2008; 63:526–31.
Article7. Dooms C, Tournoy KG, Schuurbiers O, Decaluwe H, De Ryck F, Verhagen A, et al. Endosonography for mediastinal nodal staging of clinical N1 non-small cell lung cancer: a prospective multicenter study. Chest. 2015; 147:209–15.
Article8. Kim BG, Cho JH, Shin SH, Lee K, Um SW, Kim H, et al. Diagnostic performance of endosonography to detect mediastinal lymph node metastasis in patients with radiological N1 non-small cell lung cancer. Cancer Res Treat. 2023; 55:832–40.
Article9. Sakairi Y, Hoshino H, Fujiwara T, Nakajima T, Yasufuku K, Yoshida S, et al. Validation of EBUS-TBNA-integrated nodal staging in potentially node-positive non-small cell lung cancer. Gen Thorac Cardiovasc Surg. 2013; 61:522–7.
Article10. Naur TM, Konge L, Clementsen PF. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of patients with non-small cell lung cancer without mediastinal involvement at positron emission tomography-computed tomography. Respiration. 2017; 94:279–84.
Article11. Bousema JE, Aarts MJ, Dijkgraaf MG, Annema JT, van den Broek FJ. Trends in mediastinal nodal staging and its impact on unforeseen N2 and survival in lung cancer. Eur Respir J. 2021; 57:2001549.
Article12. Ost DE, Niu J, Zhao H, Grosu HB, Giordano SH. Quality gaps and comparative effectiveness in lung cancer staging and diagnosis. Chest. 2020; 157:1322–45.
Article13. Bousema JE, Heineman DJ, Dijkgraaf MG, Annema JT, van den Broek FJ. Adherence to the mediastinal staging guideline and unforeseen N2 disease in patients with resectable non-small cell lung cancer: Nationwide results from the Dutch Lung Cancer Audit - Surgery. Lung Cancer. 2020; 142:51–8.
Article14. Shin SH, Jeong BH, Jhun BW, Yoo H, Lee K, Kim H, et al. The utility of endosonography for mediastinal staging of non-small cell lung cancer in patients with radiological N0 disease. Lung Cancer. 2020; 139:151–6.
Article15. Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016; 11:39–51.16. Giroux DJ, Rami-Porta R, Chansky K, Crowley JJ, Groome PA, Postmus PE, et al. The IASLC Lung Cancer Staging Project: data elements for the prospective project. J Thorac Oncol. 2009; 4:679–83.
Article17. Shin SH, Jeong DY, Lee KS, Cho JH, Choi YS, Lee K, et al. Which definition of a central tumour is more predictive of occult mediastinal metastasis in nonsmall cell lung cancer patients with radiological N0 disease? Eur Respir J. 2019; 53:1801508.
Article18. Kim HK, Choi YS, Kim K, Shim YM, Park K, Ahn YC, et al. Outcomes of mediastinoscopy and surgery with or without neoadjuvant therapy in patients with non-small cell lung cancer who are N2 negative on positron emission tomography and computed tomography. J Thorac Oncol. 2011; 6:336–42.
Article19. Lee KJ, Suh GY, Chung MP, Kim H, Kwon OJ, Han J, et al. Combined endobronchial and transesophageal approach of an ultrasound bronchoscope for mediastinal staging of lung cancer. PLoS One. 2014; 9:e91893.
Article20. MA IJ, Ten Broek RP, Wiering B, Hekma E, de Roos MA. Oncological outcomes of unsuspected pN2 in patients with non-small-cell lung cancer: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg. 2021; 32:727–36.
Article21. Boada M, Sanchez-Lorente D, Libreros A, Lucena CM, Marrades R, Sanchez M, et al. Is invasive mediastinal staging necessary in intermediate risk patients with negative PET/CT? J Thorac Dis. 2020; 12:3976–86.
Article22. Obiols C, Call S, Rami-Porta R, Trujillo-Reyes JC, Saumench R, Iglesias M, et al. Survival of patients with unsuspected pN2 non-small cell lung cancer after an accurate preoperative mediastinal staging. Ann Thorac Surg. 2014; 97:957–64.
Article23. Yasufuku K, Nakajima T, Chiyo M, Sekine Y, Shibuya K, Fujisawa T. Endobronchial ultrasonography: current status and future directions. J Thorac Oncol. 2007; 2:970–9.
Article24. Ernst A, Eberhardt R, Krasnik M, Herth FJ. Efficacy of endobronchial ultrasound-guided transbronchial needle aspiration of hilar lymph nodes for diagnosing and staging cancer. J Thorac Oncol. 2009; 4:947–50.
Article25. Chaft JE, Shyr Y, Sepesi B, Forde PM. Preoperative and postoperative systemic therapy for operable non-small-cell lung cancer. J Clin Oncol. 2022; 40:546–55.
Article26. Cho HJ, Kim SR, Kim HR, Han JO, Kim YH, Kim DK, et al. Modern outcome and risk analysis of surgically resected occult N2 non-small cell lung cancer. Ann Thorac Surg. 2014; 97:1920–5.
Article27. Bille A, Woo KM, Ahmad U, Rizk NP, Jones DR. Incidence of occult pN2 disease following resection and mediastinal lymph node dissection in clinical stage I lung cancer patients. Eur J Cardiothorac Surg. 2017; 51:674–9.
Article28. Lee PC, Port JL, Korst RJ, Liss Y, Meherally DN, Altorki NK. Risk factors for occult mediastinal metastases in clinical stage I non-small cell lung cancer. Ann Thorac Surg. 2007; 84:177–81.
Article29. Kuijvenhoven JC, Korevaar DA, Tournoy KG, Malfait TL, Dooms C, Rintoul RC, et al. Five-year survival after endosonography vs mediastinoscopy for mediastinal nodal staging of lung cancer. JAMA. 2016; 316:1110–2.
Article30. Bousema JE, van Dorp M, Noyez V, Dijkgraaf MG, Annema JT, van den Broek FJ. Unforeseen N2 disease after negative endosonography findings with or without confirmatory mediastinoscopy in resectable non-small cell lung cancer: a systematic review and meta-analysis. J Thorac Oncol. 2019; 14:979–92.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnostic Performance of Endosonography to Detect Mediastinal Lymph Node Metastasis in Patients with Radiological N1 Non–Small Cell Lung Cancer
- Clinical Significance of the Aortic Node in Non-small Cell Lung Cancer of the Left Upper Lobe
- Postoperative Radiotherapy for Non-Small Cell Lung Cancer
- Adjuvant Chemotherapy for Completely Resected Non-Small Cell Lung Cancer
- Definitive Radiotherapy of Non-Small Cell Lung Cancer