Anat Cell Biol.
2024 Mar;57(1):70-84. 10.5115/acb.23.193.
Exploring amygdala structural changes and signaling pathways in postmortem brains: consequences of long-term methamphetamine addiction
- Affiliations
-
- 1Hearing Disorders Research Center, Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 2Laser Application in Medical Sciences Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 3Functional Neurosurgery Research Center, Shohada Tajrish Comprehensive Neurosurgical Center of Excellence, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 4Department of Anatomical Sciences, School of Medicine, Cellular and Molecular Research Center, Birjand University of Medical Sciences, Birjand, Iran
- 5Private Practice, Bradford, ON, Canada
- 6Department of Biology and Anatomical Sciences, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 7Iranian Legal Medicine Organization, Tehran, Iran
- 8Cellular and Molecular Research Center, Research Institute for Non-Communicable Diseases, Qazvin University of Medical Sciences, Qazvin, Iran
- KMID: 2554242
- DOI: http://doi.org/10.5115/acb.23.193
Abstract
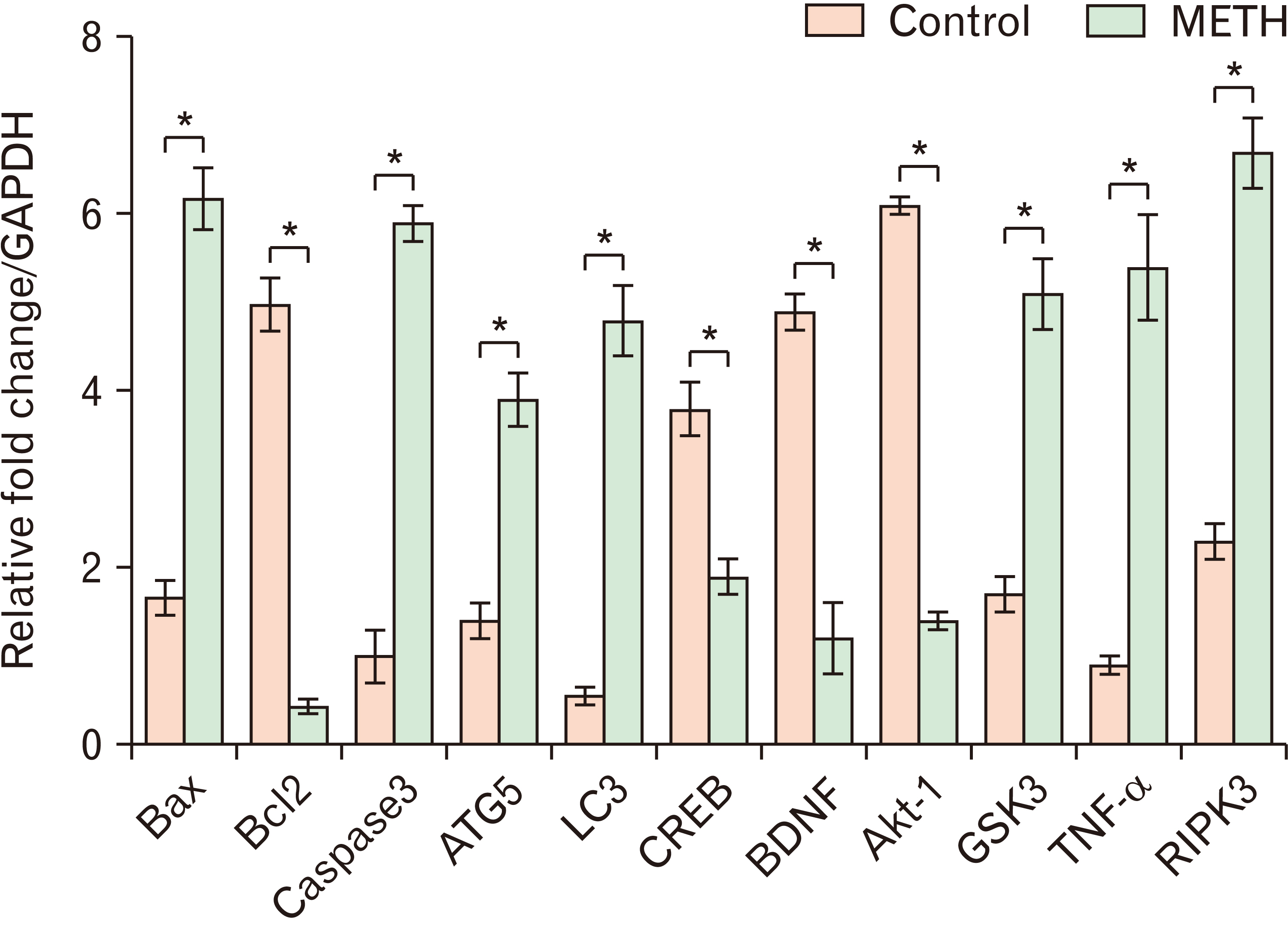
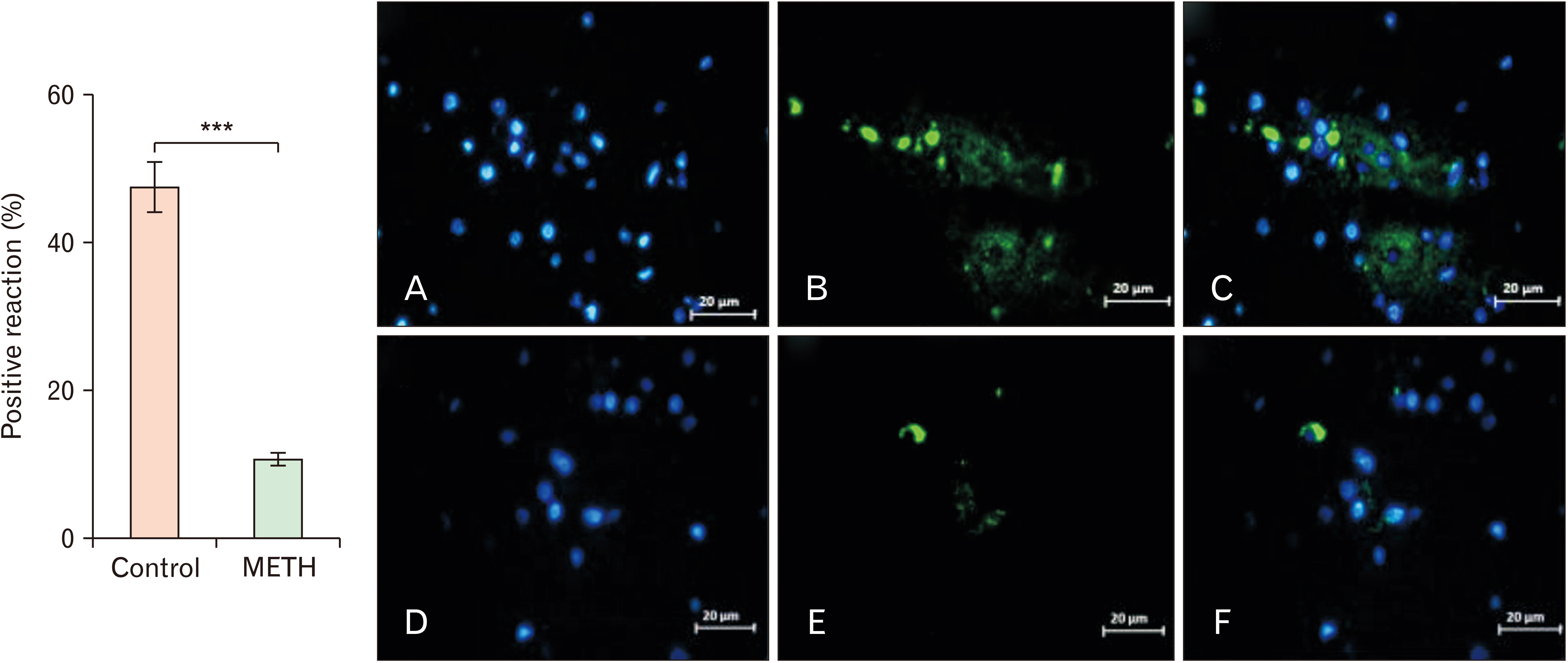
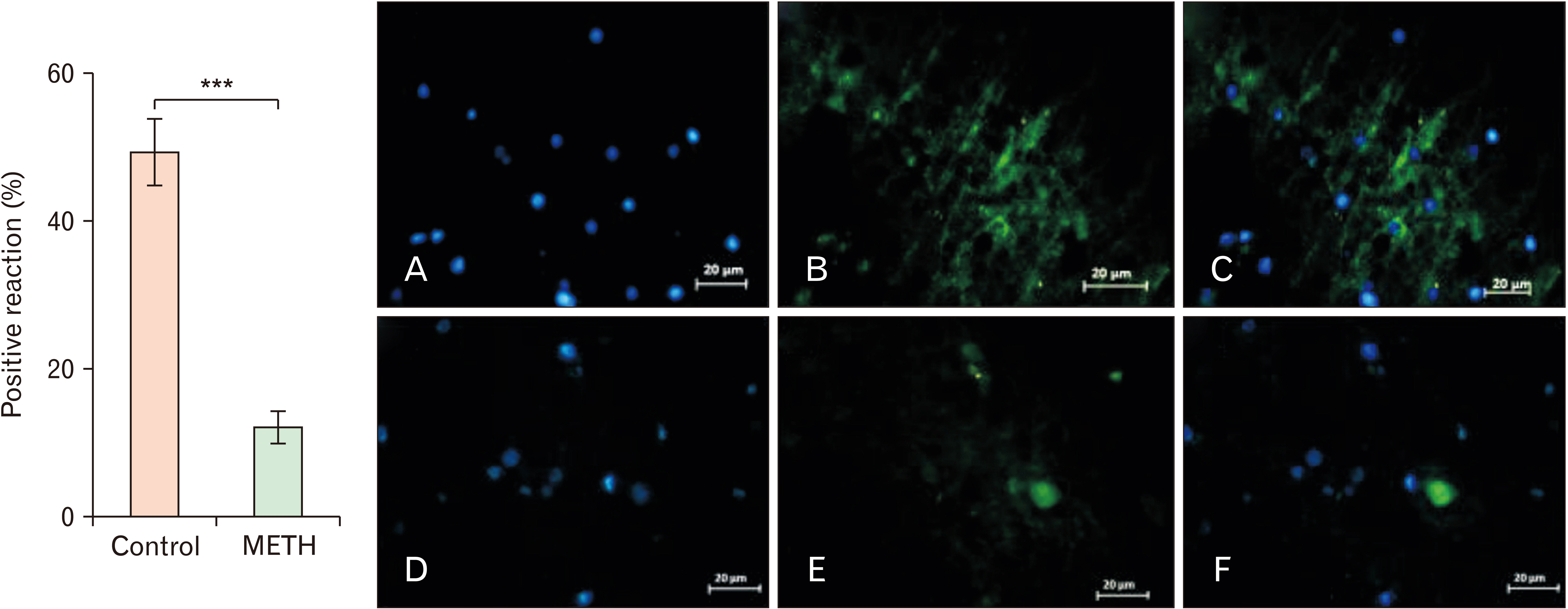
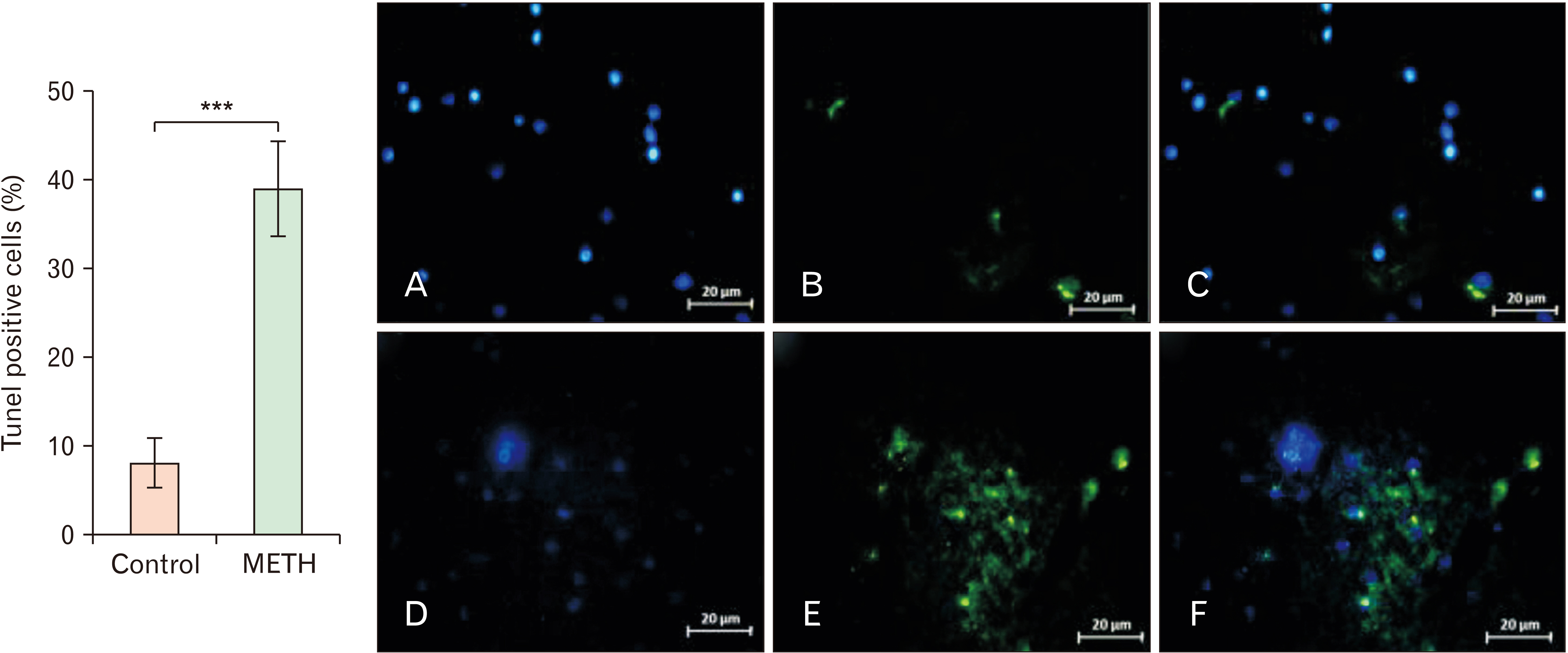
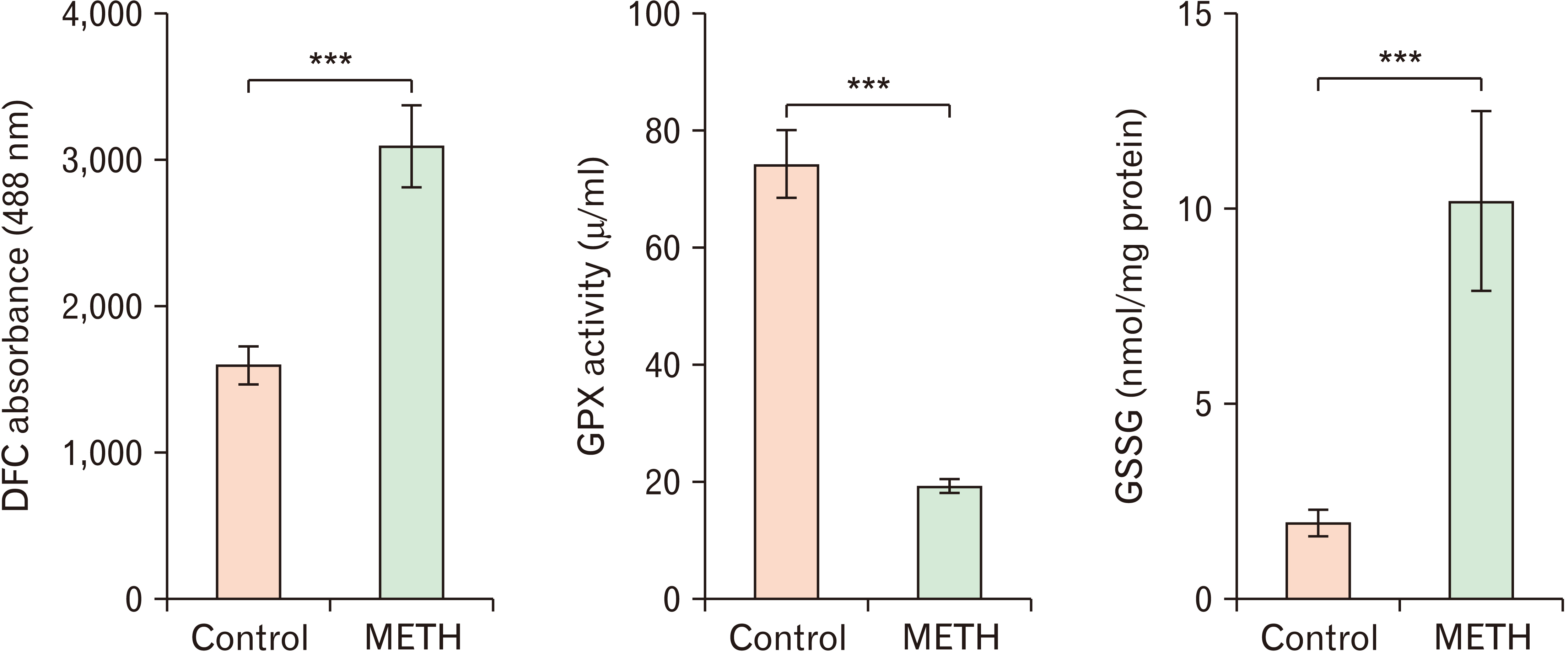
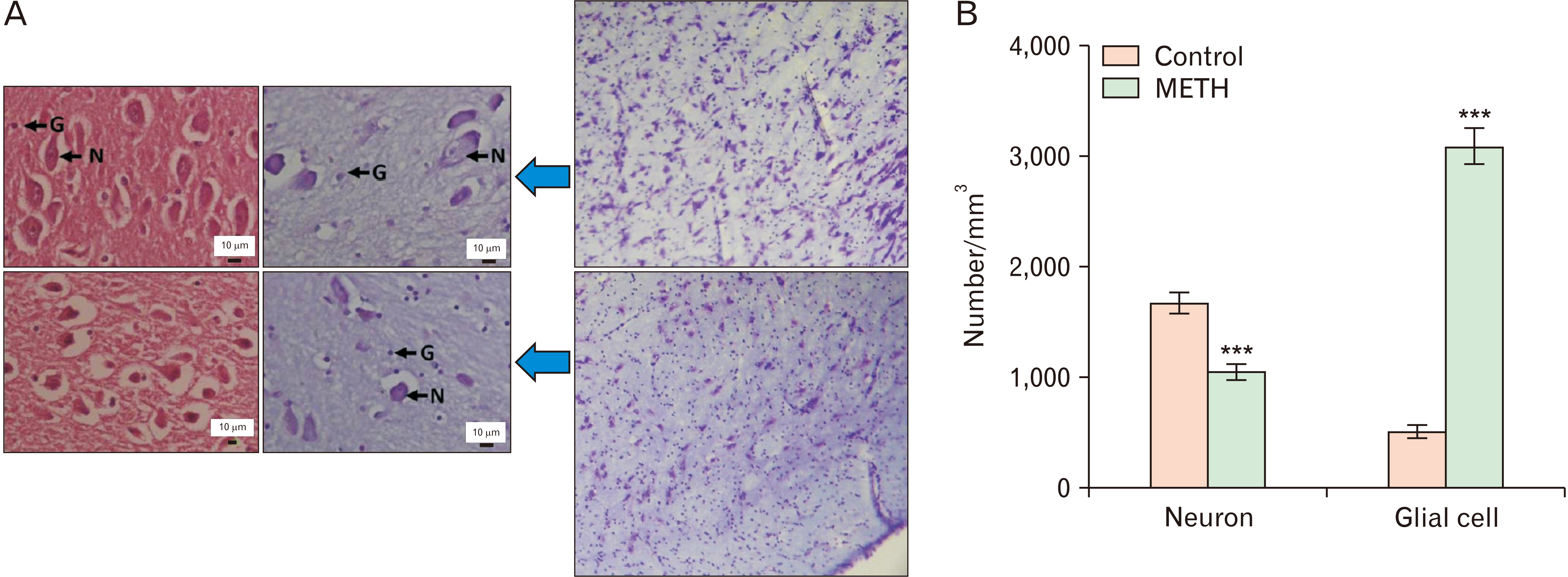
- Methamphetamine (METH) can potentially disrupt neurotransmitters activities in the central nervous system (CNS) and cause neurotoxicity through various pathways. These pathways include increased production of reactive nitrogen and oxygen species, hypothermia, and induction of mitochondrial apoptosis. In this study, we investigated the long-term effects of METH addiction on the structural changes in the amygdala of postmortem human brains and the involvement of the brain- cAMP response element-binding protein/brain-derived neurotrophic factor (CREB/BDNF) and Akt-1/GSK3 signaling pathways. We examined ten male postmortem brains, comparing control subjects with chronic METH users, using immunohistochemistry, real-time polymerase chain reaction (to measure levels of CREB, BDNF, Akt-1, GSK3, and tumor necrosis factor-α [TNF-α]), Tunnel assay, stereology, and assays for reactive oxygen species (ROS), glutathione disulfide (GSSG), and glutathione peroxidase (GPX). The findings revealed that METH significantly reduced the expression of BDNF, CREB, Akt-1, and GPX while increasing the levels of GSSG, ROS, RIPK3, GSK3, and TNF-α. Furthermore, METH-induced inflammation and neurodegeneration in the amygdala, with ROS production mediated by the CREB/BDNF and Akt-1/GSK3 signaling pathways.
Keyword
Figure
Reference
-
References
1. Behl T, Makkar R, Sehgal A, Singh S, Sharma N, Zengin G, Bungau S, Andronie-Cioara FL, Munteanu MA, Brisc MC, Uivarosan D, Brisc C. 2021; Current trends in neurodegeneration: cross talks between oxidative stress, cell death, and inflammation. Int J Mol Sci. 22:7432. DOI: 10.3390/ijms22147432. PMID: 34299052. PMCID: PMC8306752.
Article2. Darabi S, Tiraihi T, Ruintan A, Abbaszadeh HA, Delshad A, Taheri T. 2013; Polarized neural stem cells derived from adult bone marrow stromal cells develop a rosette-like structure. In Vitro Cell Dev Biol Anim. 49:638–52. DOI: 10.1007/s11626-013-9628-y. PMID: 23771792.
Article3. Pourgholaminejad A, Tahmasebinia F. Mitoma H, Manto M, editors. The role of Th17 cells in immunopathogenesis of neuroinflammatory disorders. Neuroimmune Diseases: From Cells to the Living Brain. Springer;2019. p. 83–107. DOI: 10.1007/978-3-030-19515-1_3.
Article4. Mahmoudiasl GR, Abbaszadeh HA, Rezaei-Tavirani M, Abdollahifar MA, Khoramgah MS, Niknazar S, Darabi S, Roozbahany NA. 2019; Nod-like receptor protein 3 and nod-like receptor protein 1 inflammasome activation in the hippocampal region of postmortem methamphetamine chronic user. Bratisl Lek Listy. 120:769–76. DOI: 10.4149/BLL_2019_129. PMID: 31663353.
Article5. Khoshsirat S, Khoramgah MS, Mahmoudiasl GR, Rezaei-Tavirani M, Abdollahifar MA, Tahmasebinia F, Darabi S, Niknazar S, Abbaszadeh HA. 2020; LC3 and ATG5 overexpression and neuronal cell death in the prefrontal cortex of postmortem chronic methamphetamine users. J Chem Neuroanat. 107:101802. DOI: 10.1016/j.jchemneu.2020.101802. PMID: 32416129.
Article6. Rasti Boroojeni F, Mashayekhan S, Abbaszadeh HA, Ansarizadeh M, Khoramgah MS, Rahimi Movaghar V. 2020; Bioinspired nanofiber scaffold for differentiating bone marrow-derived neural stem cells to oligodendrocyte-like cells: design, fabrication, and characterization. Int J Nanomedicine. 15:3903–20. DOI: 10.2147/IJN.S248509. PMID: 32606657. PMCID: PMC7293409.7. Cadet JL, Krasnova IN. 2009; Molecular bases of methamphetamine-induced neurodegeneration. Int Rev Neurobiol. 88:101–19. DOI: 10.1016/S0074-7742(09)88005-7. PMID: 19897076.
Article8. İvecen B, Gokdemir O. 2022; Methamphetamine addiction. Dahuder Med J. 2:98–101. DOI: 10.56016/dahudermj.1184367.
Article9. Prakash MD, Tangalakis K, Antonipillai J, Stojanovska L, Nurgali K, Apostolopoulos V. 2017; Methamphetamine: effects on the brain, gut and immune system. Pharmacol Res. 120:60–7. DOI: 10.1016/j.phrs.2017.03.009. PMID: 28302577.
Article10. Chavda V, Chaurasia B, Umana GE, Tomasi SO, Lu B, Montemurro N. 2022; Narcolepsy-a neuropathological obscure sleep disorder: a narrative review of current literature. Brain Sci. 12:1473. DOI: 10.3390/brainsci12111473. PMID: 36358399. PMCID: PMC9688775.
Article11. Rusyniak DE. 2013; Neurologic manifestations of chronic methamphetamine abuse. Psychiatr Clin North Am. 36:261–75. DOI: 10.1016/j.psc.2013.02.005. PMID: 23688691. PMCID: PMC3764482.
Article12. Compton WM, Volkow ND. 2006; Abuse of prescription drugs and the risk of addiction. Drug Alcohol Depend. 83 Suppl 1:S4–7. DOI: 10.1016/j.drugalcdep.2005.10.020. PMID: 16563663.
Article13. Downey LA, Loftis JM. 2014; Altered energy production, lowered antioxidant potential, and inflammatory processes mediate CNS damage associated with abuse of the psychostimulants MDMA and methamphetamine. Eur J Pharmacol. 727:125–9. DOI: 10.1016/j.ejphar.2014.01.032. PMID: 24485894. PMCID: PMC3970781.
Article14. Varner KJ, Ogden BA, Delcarpio J, Meleg-Smith S. 2002; Cardiovascular responses elicited by the "binge" administration of methamphetamine. J Pharmacol Exp Ther. 301:152–9. DOI: 10.1124/jpet.301.1.152. PMID: 11907169.
Article15. Tehrani AM, Boroujeni ME, Aliaghaei A, Feizi MAH, Safaralizadeh R. 2019; Methamphetamine induces neurotoxicity-associated pathways and stereological changes in prefrontal cortex. Neurosci Lett. 712:134478. DOI: 10.1016/j.neulet.2019.134478. PMID: 31491463.
Article16. Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. 2001; Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 158:377–82. DOI: 10.1176/appi.ajp.158.3.377. PMID: 11229977.
Article17. Shrestha P, Katila N, Lee S, Seo JH, Jeong JH, Yook S. 2022; Methamphetamine induced neurotoxic diseases, molecular mechanism, and current treatment strategies. Biomed Pharmacother. 154:113591. DOI: 10.1016/j.biopha.2022.113591. PMID: 36007276.
Article18. Hong SJ, Zhang D, Zhang LH, Yang P, Wan J, Yu Y, Wang TH, Feng ZT, Li LH, Yew DT. 2015; Expression of dopamine transporter in the different cerebral regions of methamphetamine-dependent rats. Hum Exp Toxicol. 34:707–17. DOI: 10.1177/0960327114555929. PMID: 25504685.
Article19. Yuan J, Lv R, Robert Brašić J, Han M, Liu X, Wang Y, Zhang G, Liu C, Li Y, Deng Y. 2014; Dopamine transporter dysfunction in Han Chinese people with chronic methamphetamine dependence after a short-term abstinence. Psychiatry Res. 221:92–6. DOI: 10.1016/j.pscychresns.2013.11.005. PMID: 24314908.
Article20. London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, Bramen J, Shinn AK, Miotto K, Learn J, Dong Y, Matochik JA, Kurian V, Newton T, Woods R, Rawson R, Ling W. 2004; Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry. 61:73–84. DOI: 10.1001/archpsyc.61.1.73. PMID: 14706946.
Article21. Moszczynska A, Fitzmaurice P, Ang L, Kalasinsky KS, Schmunk GA, Peretti FJ, Aiken SS, Wickham DJ, Kish SJ. 2004; Why is parkinsonism not a feature of human methamphetamine users? Brain. 127(Pt 2):363–70. DOI: 10.1093/brain/awh046. PMID: 14645148.
Article22. Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. 2004; Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 24:6028–36. DOI: 10.1523/JNEUROSCI.0713-04.2004. PMID: 15229250. PMCID: PMC6729247.
Article23. Miller DB, O'Callaghan JP. 2003; Elevated environmental temperature and methamphetamine neurotoxicity. Environ Res. 92:48–53. DOI: 10.1016/S0013-9351(02)00051-8. PMID: 12706754.
Article24. Jayanthi S, Deng X, Ladenheim B, McCoy MT, Cluster A, Cai NS, Cadet JL. 2005; Calcineurin/NFAT-induced up-regulation of the Fas ligand/Fas death pathway is involved in methamphetamine-induced neuronal apoptosis. Proc Natl Acad Sci U S A. 102:868–73. DOI: 10.1073/pnas.0404990102. PMID: 15644446. PMCID: PMC545515.
Article25. Kiyatkin EA, Sharma HS. 2009; Permeability of the blood-brain barrier depends on brain temperature. Neuroscience. 161:926–39. DOI: 10.1016/j.neuroscience.2009.04.004. PMID: 19362131. PMCID: PMC2694729.
Article26. Nopparat C, Porter JE, Ebadi M, Govitrapong P. 2010; The mechanism for the neuroprotective effect of melatonin against methamphetamine-induced autophagy. J Pineal Res. 49:382–9. DOI: 10.1111/j.1600-079X.2010.00805.x. PMID: 20738755.
Article27. Huang X, Chen YY, Shen Y, Cao X, Li A, Liu Q, Li Z, Zhang LB, Dai W, Tan T, Arias-Carrion O, Xue YX, Su H, Yuan TF. 2017; Methamphetamine abuse impairs motor cortical plasticity and function. Mol Psychiatry. 22:1274–81. DOI: 10.1038/mp.2017.143. PMID: 28831198. PMCID: PMC5582165.
Article28. Salo R, Fassbender C, Buonocore MH, Ursu S. 2013; Behavioral regulation in methamphetamine abusers: an fMRI study. Psychiatry Res. 211:234–8. DOI: 10.1016/j.pscychresns.2012.10.003. PMID: 23149023. PMCID: PMC3594424.
Article29. Morales AM, Lee B, Hellemann G, O'Neill J, London ED. 2012; Gray-matter volume in methamphetamine dependence: cigarette smoking and changes with abstinence from methamphetamine. Drug Alcohol Depend. 125:230–8. DOI: 10.1016/j.drugalcdep.2012.02.017. PMID: 22445480. PMCID: PMC3427723.
Article30. Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TD, Heaton RK, Ellis RJ, Grant I. 2005; Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry. 162:1461–72. Erratum in: Am J Psychiatry 2005;162:1774. DOI: 10.1176/appi.ajp.162.8.1461. PMID: 16055767.
Article31. Gonçalves J, Baptista S, Martins T, Milhazes N, Borges F, Ribeiro CF, Malva JO, Silva AP. 2010; Methamphetamine-induced neuroinflammation and neuronal dysfunction in the mice hippocampus: preventive effect of indomethacin. Eur J Neurosci. 31:315–26. DOI: 10.1111/j.1460-9568.2009.07059.x. PMID: 20074221.
Article32. Tabatabaei Mirakabad FS, Khoramgah MS, Abdollahifar MA, Tehrani AS, Rezaei-Tavirani M, Niknazar S, Tahmasebinia F, Mahmoudiasl GR, Khoshsirat S, Abbaszadeh HA. 2021; NUPR1-CHOP experssion, autophagosome formation and apoptosis in the postmortem striatum of chronic methamphetamine user. J Chem Neuroanat. 114:101942. DOI: 10.1016/j.jchemneu.2021.101942. PMID: 33675952.33. Roohbakhsh A, Shirani K, Karimi G. 2016; Methamphetamine-induced toxicity: the role of autophagy? Chem Biol Interact. 260:163–7. DOI: 10.1016/j.cbi.2016.10.012. PMID: 27746146.
Article34. Mata MM, Napier TC, Graves SM, Mahmood F, Raeisi S, Baum LL. 2015; Methamphetamine decreases CD4 T cell frequency and alters pro-inflammatory cytokine production in a model of drug abuse. Eur J Pharmacol. 752:26–33. DOI: 10.1016/j.ejphar.2015.02.002. PMID: 25678251. PMCID: PMC4396630.
Article35. Kohno M, Loftis JM, Huckans M, Dennis LE, McCready H, Hoffman WF. 2018; The relationship between interleukin-6 and functional connectivity in methamphetamine users. Neurosci Lett. 677:49–54. DOI: 10.1016/j.neulet.2018.04.037. PMID: 29689344. PMCID: PMC5971003.
Article36. Guo D, Huang X, Xiong T, Wang X, Zhang J, Wang Y, Liang J. 2022; Molecular mechanisms of programmed cell death in methamphetamine-induced neuronal damage. Front Pharmacol. 13:980340. DOI: 10.3389/fphar.2022.980340. PMID: 36059947. PMCID: PMC9428134.
Article37. Wu CW, Ping YH, Yen JC, Chang CY, Wang SF, Yeh CL, Chi CW, Lee HC. 2007; Enhanced oxidative stress and aberrant mitochondrial biogenesis in human neuroblastoma SH-SY5Y cells during methamphetamine induced apoptosis. Toxicol Appl Pharmacol. 220:243–51. DOI: 10.1016/j.taap.2007.01.011. PMID: 17350664.
Article38. Gholami M, Hozuri F, Abdolkarimi S, Mahmoudi M, Motaghinejad M, Safari S, Sadr S. 2021; Pharmacological and molecular evidence of neuroprotective curcumin effects against biochemical and behavioral sequels caused by methamphetamine: possible function of CREB-BDNF signaling pathway. Basic Clin Neurosci. 12:325–38. DOI: 10.32598/bcn.2021.1176.3. PMID: 34917292. PMCID: PMC8666919.
Article39. Motaghinejad M, Motevalian M, Babalouei F, Abdollahi M, Heidari M, Madjd Z. 2017; Possible involvement of CREB/BDNF signaling pathway in neuroprotective effects of topiramate against methylphenidate induced apoptosis, oxidative stress and inflammation in isolated hippocampus of rats: molecular, biochemical and histological evidences. Brain Res Bull. 132:82–98. DOI: 10.1016/j.brainresbull.2017.05.011. PMID: 28552672.
Article40. Motaghinejad M, Mashayekh R, Motevalian M, Safari S. 2021; The possible role of CREB-BDNF signaling pathway in neuroprotective effects of minocycline against alcohol-induced neurodegeneration: molecular and behavioral evidences. Fundam Clin Pharmacol. 35:113–30. DOI: 10.1111/fcp.12584. PMID: 32579730.
Article41. Feizipour S, Sobhani S, Mehrafza S, Gholami M, Motaghinejad M, Motevalian M, Safari S, Davoudizadeh R. 2020; Selegiline acts as neuroprotective agent against methamphetamine-prompted mood and cognitive related behavior and neurotoxicity in rats: involvement of CREB/BDNF and Akt/GSK3 signal pathways. Iran J Basic Med Sci. 23:606–15.42. Keshavarzi S, Kermanshahi S, Karami L, Motaghinejad M, Motevalian M, Sadr S. 2019; Protective role of metformin against methamphetamine induced anxiety, depression, cognition impairment and neurodegeneration in rat: the role of CREB/BDNF and Akt/GSK3 signaling pathways. Neurotoxicology. 72:74–84. DOI: 10.1016/j.neuro.2019.02.004. PMID: 30742852.
Article43. Darabi S, Noori-Zadeh A, Rajaei F, Abbaszadeh HA, Bakhtiyari S, Roozbahany NA. 2018; SMER28 attenuates dopaminergic toxicity mediated by 6-hydroxydopamine in the rats via modulating oxidative burdens and autophagy-related parameters. Neurochem Res. 43:2313–23. DOI: 10.1007/s11064-018-2652-2. PMID: 30288644.
Article44. Darabi S, Tiraihi T, Noori-zadeh A, Rajaei F, Darabi L, Abbaszadeh H. 2017; Creatine and retinoic acid effects on the induction of autophagy and differentiation of adipose tissue-derived stem cells into GABAergic-like neurons. J Babol Univ Med Sci. 19:41–9.45. Abbaszadeh HA, Tiraihi T, Delshad AR, Saghedi Zadeh M, Taheri T. 2013; Bone marrow stromal cell transdifferentiation into oligodendrocyte-like cells using triiodothyronine as a inducer with expression of platelet-derived growth factor α as a maturity marker. Iran Biomed J. 17:62–70.46. Moghimi N, Eslami Farsani B, Ghadipasha M, Mahmoudiasl GR, Piryaei A, Aliaghaei A, Abdi S, Abbaszadeh HA, Abdollahifar MA, Forozesh M. 2021; COVID-19 disrupts spermatogenesis through the oxidative stress pathway following induction of apoptosis. Apoptosis. 26:415–30. DOI: 10.1007/s10495-021-01680-2. PMID: 34076792. PMCID: PMC8170653.
Article47. Shaerzadeh F, Streit WJ, Heysieattalab S, Khoshbouei H. 2018; Methamphetamine neurotoxicity, microglia, and neuroinflammation. J Neuroinflammation. 15:341. DOI: 10.1186/s12974-018-1385-0. PMID: 30541633. PMCID: PMC6292109.
Article48. Castellano P, Nwagbo C, Martinez LR, Eugenin EA. 2016; Methamphetamine compromises gap junctional communication in astrocytes and neurons. J Neurochem. 137:561–75. DOI: 10.1111/jnc.13603. PMID: 26953131. PMCID: PMC5050922.
Article49. Mayr B, Montminy M. 2001; Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2:599–609. DOI: 10.1038/35085068. PMID: 11483993.
Article50. Yang G, Li J, Peng Y, Shen B, Li Y, Liu L, Wang C, Xu Y, Lin S, Zhang S, Tan Y, Zhang H, Zeng X, Li Q, Lu G. 2022; Ginsenoside Rb1 attenuates methamphetamine (METH)-induced neurotoxicity through the NR2B/ERK/CREB/BDNF signalings in vitro and in vivo models. J Ginseng Res. 46:426–34. Erratum in: J Ginseng Res 2023;47:349-51. DOI: 10.1016/j.jgr.2023.01.001. PMID: 36926603. PMCID: PMC10014215.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of MK-801 on Methamphetamine - Induced Dopaminergic Neurotoxicity: Long-Term Attenuation of Methamphetamine - Induced Dopamine Release
- Postmortem Findings in Two Cases of Chronic Methamphetamine Abuse
- Methamphetamine-Induced Neuronal Damage: Neurotoxicity and Neuroinflammation
- A case of methamphetamine intoxication in an adolescent
- Lethal Hemorrhage Following Laparoscopic Cholecystectomy Complicated with Medicolegal Issues