J Rhinol.
2024 Mar;31(1):1-7. 10.18787/jr.2024.00006.
Efficacy of Platelet-Rich Plasma in the Treatment of Persistent Olfactory Impairment After COVID-19: A Systematic Review and Meta-Analysis
- Affiliations
-
- 1Department of Otolaryngology-Head and Neck Surgery, Bucheon Saint Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea
- 2Department of Otolaryngology-Head and Neck Surgery, Seoul Saint Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea
- KMID: 2554084
- DOI: http://doi.org/10.18787/jr.2024.00006
Abstract
- Background and Objectives
This study aimed to evaluate the impact of topical platelet-rich plasma (PRP) injection on persistent refractory olfactory dysfunction after COVID-19 infection.
Methods
A systematic review was conducted, focusing on studies that compared the efficacy of topical PRP treatment with a control group (receiving either placebo or no treatment) in ameliorating olfactory dysfunction. Pre- and post-treatment comparisons were evaluated, along with a subgroup analysis of olfactory function evaluation.
Results
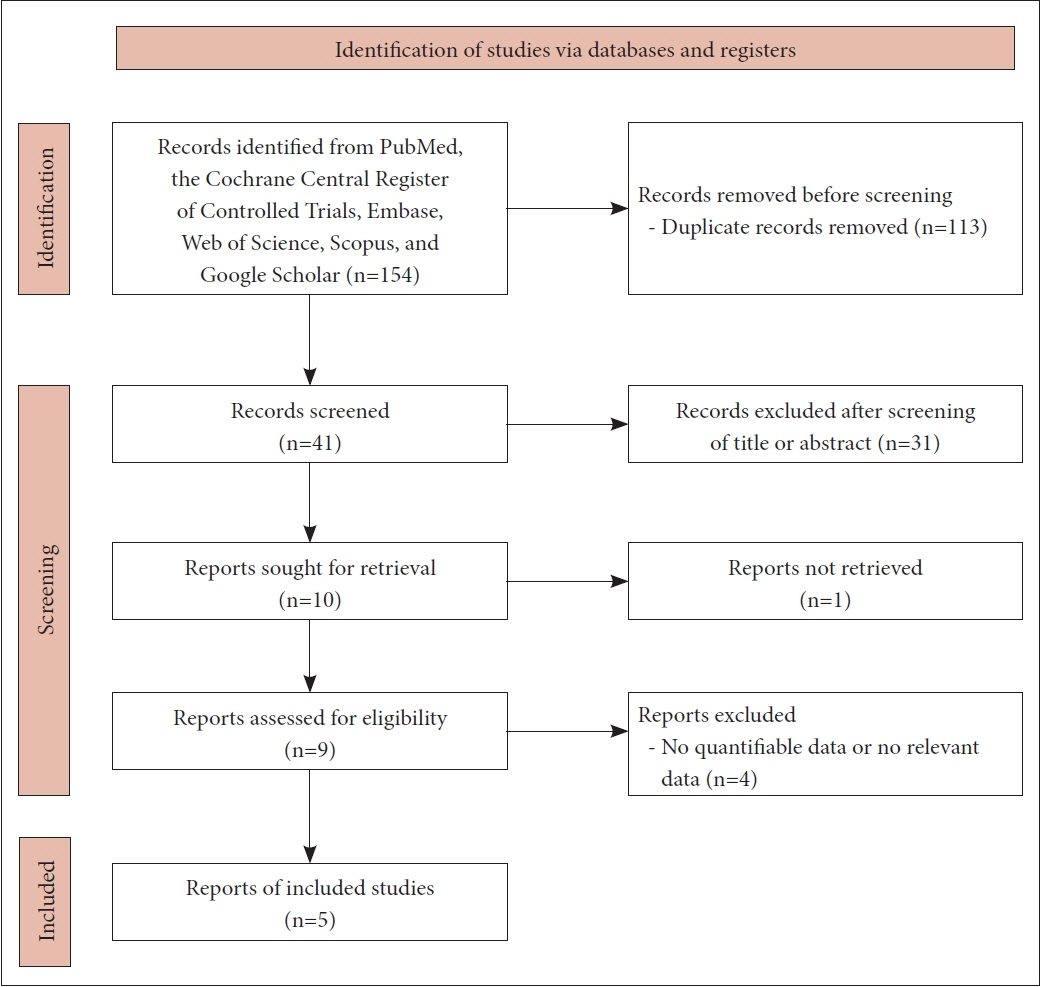
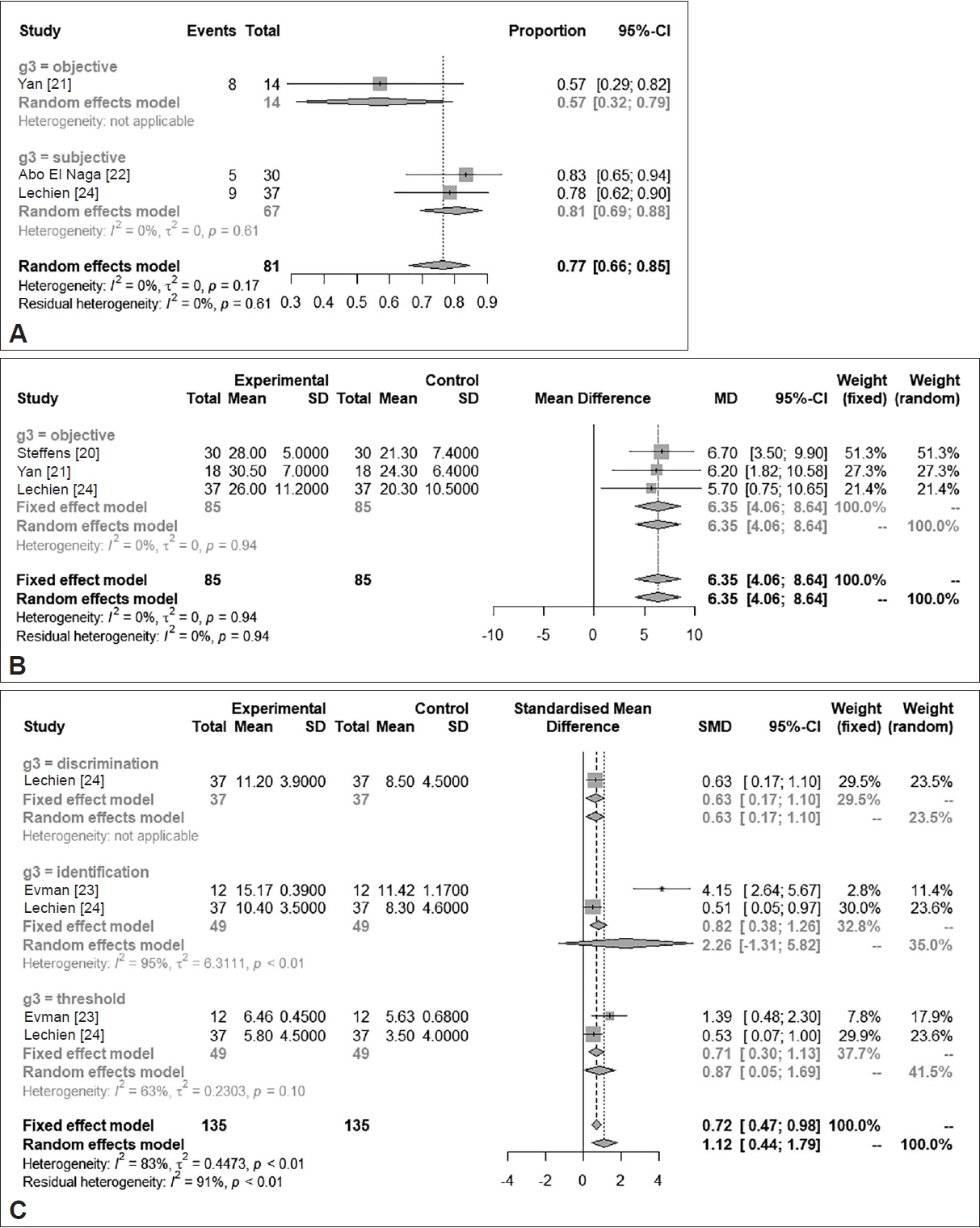
The analysis revealed a significant improvement in olfactory scores between 1 to 3 months post-treatment (standardized mean difference=1.4376; 95% confidence interval [CI]=0.5934–2.2818; I2=84.1%) in the treatment group compared to the control group. Moreover, a notable disparity was observed between the two groups in the incidence of substantial recovery from anosmia or hyposmia (odds ratio=8.6639; 95% CI=2.9752–25.2292; I2=0.0%). PRP treatment led to a clinically significant increase in the threshold, discrimination, and identification (TDI) score for the Sniffin’ Sticks test by >5.5 (minimum clinically significant difference; mean difference, 6.3494; 95% CI=4.0605–8.6384; I2=0.0%), as confirmed by verified examinations. The odds ratio for significant improvement among patients after treatment was determined to be 0.7654 (95% CI=0.6612–0.8451). Furthermore, all TDI subdomains exhibited significant and comparable improvements post-treatment.
Conclusion
This meta-analysis indicates that the injection of PRP into the olfactory fissure or surrounding mucosal areas is an effective treatment for persistent refractory olfactory dysfunction.
Figure
Reference
-
References
1. Croy I, Nordin S, Hummel T. Olfactory disorders and quality of life--an updated review. Chem Senses. 2014; 39(3):185–94.
Article2. Hummel T, Whitcroft KL, Andrews P, Altundag A, Cinghi C, Costanzo RM, et al. Position paper on olfactory dysfunction. Rhinol Suppl. 2017; 54(26):1–30.
Article3. Whitcroft KL, Altundag A, Balungwe P, Boscolo-Rizzo P, Douglas R, Enecilla MLB, et al. Position paper on olfactory dysfunction: 2023. Rhinology. 2023; Jul. 16. [Epub]. Available from: https://www.rhinologyjournal.com/Abstract.php?id=3097.4. Kim DH, Kim SW, Hwang SH, Kim BG, Kang JM, Cho JH, et al. Prognosis of olfactory dysfunction according to etiology and timing of treatment. Otolaryngol Head Neck Surg. 2017; 156(2):371–7.
Article5. Cavazzana A, Larsson M, Münch M, Hähner A, Hummel T. Postinfectious olfactory loss: a retrospective study on 791 patients. Laryngoscope. 2018; 128(1):10–5.
Article6. Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011; 39(10):2135–40.
Article7. Anjayani S, Wirohadidjojo YW, Adam AM, Suwandi D, Seweng A, Amiruddin MD. Sensory improvement of leprosy peripheral neuropathy in patients treated with perineural injection of platelet-rich plasma. Int J Dermatol. 2014; 53(1):109–13.
Article8. Kim DH, Lee MH, Lee J, Song EA, Kim SW, Kim SW. Platelet-rich plasma injection in patients with atrophic rhinitis. ORL J Otorhinolaryngol Relat Spec. 2021; 83(2):104–11.
Article9. Raeissadat SA, Karimzadeh A, Hashemi M, Bagherzadeh L. Safety and efficacy of platelet-rich plasma in treatment of carpal tunnel syndrome; a randomized controlled trial. BMC Musculoskelet Disord. 2018; 19(1):49.
Article10. Farrag TY, Lehar M, Verhaegen P, Carson KA, Byrne PJ. Effect of platelet rich plasma and fibrin sealant on facial nerve regeneration in a rat model. Laryngoscope. 2007; 117(1):157–65.
Article11. Ikumi A, Hara Y, Yoshioka T, Kanamori A, Yamazaki M. Effect of local administration of platelet-rich plasma (PRP) on peripheral nerve regeneration: an experimental study in the rabbit model. Microsurgery. 2018; 38(3):300–9.
Article12. Sariguney Y, Yavuzer R, Elmas C, Yenicesu I, Bolay H, Atabay K. Effect of platelet-rich plasma on peripheral nerve regeneration. J Reconstr Microsurg. 2008; 24(3):159–67.
Article13. Zheng C, Zhu Q, Liu X, Huang X, He C, Jiang L, et al. Effect of platelet-rich plasma (PRP) concentration on proliferation, neurotrophic function and migration of Schwann cells in vitro. J Tissue Eng Regen Med. 2016; 10(5):428–36.
Article14. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021; 372:n71.15. Hwang SH, Kim SW, Basurrah MA, Kim DH. Efficacy of steroid-impregnated spacers after endoscopic sinus surgery in chronic rhinosinusitis: a systematic review and meta-analysis. Clin Exp Otorhinolaryngol. 2023; 16(2):148–58.
Article16. Kim DH, Kim SW, Basurrah MA, Hwang SH. Clinical and laboratory features of various criteria of eosinophilic chronic rhinosinusitis: a systematic review and meta-analysis. Clin Exp Otorhinolaryngol. 2022; 15(3):230–46.
Article17. Mavrogeni P, Kanakopoulos A, Maihoub S, Krasznai M, Szirmai A. Anosmia treatment by platelet rich plasma injection. Int Tinnitus J. 2017; 20(2):102–5.
Article18. Yan CH, Mundy DC, Patel ZM. The use of platelet-rich plasma in treatment of olfactory dysfunction: a pilot study. Laryngoscope Investig Otolaryngol. 2020; 5(2):187–93.
Article19. Aboelmagd EA, Mohamed EF, Abdelmegeed EM, Eltahan AA. Platelet-rich plasma in the management of anosmia. Egypt J Neck Surg Otorhinolaryngol. 2021; 7(1):10–9.20. Steffens Y, Le Bon SD, Lechien J, Prunier L, Rodriguez A, Saussez S, et al. Effectiveness and safety of PRP on persistent olfactory dysfunction related to COVID-19. Eur Arch Otorhinolaryngol. 2022; 279(12):5951–3.
Article21. Yan CH, Jang SS, Lin HC, Ma Y, Khanwalkar AR, Thai A, et al. Use of platelet-rich plasma for COVID-19-related olfactory loss: a randomized controlled trial. Int Forum Allergy Rhinol. 2023; 13(6):989–97.
Article22. Abo El Naga HA, El Zaiat RS, Hamdan AM. The potential therapeutic effect of platelet-rich plasma in the treatment of post-COVID-19 parosmia. Egypt J Otolaryngol. 2022; 38(1):130.
Article23. Evman MD, Cetin ZE. Effectiveness of platelet-rich plasma on post-COVID chronic olfactory dysfunction. Rev Assoc Med Bras (1992). 2023; 69(11):e20230666.
Article24. Lechien JR, Le Bon SD, Saussez S. Platelet-rich plasma injection in the olfactory clefts of COVID-19 patients with long-term olfactory dysfunction. Eur Arch Otorhinolaryngol. 2023; 280(5):2351–8.
Article25. Shawky MA, Hadeya AM. Platelet-rich plasma in management of anosmia (single versus double injections). Indian J Otolaryngol Head Neck Surg. 2023; 75(Suppl 1):1004–8.
Article26. Sorour SS, Elhady AM, Hosny SM, Gad EMA. Assessment of olfactory dysfunction after treatment with platelet-rich plasma. Egypt J Hosp Med. 2023; 91(1):5094–9.
Article27. Stavrakas M, Karkos PD, Markou K, Grigoriadis N. Platelet-rich plasma in otolaryngology. J Laryngol Otol. 2016; 130(12):1098–102.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Potency of Platelet-Rich Plasma for Chronic Low Back Pain: A Systematic Review and Metaanalysis of Randomized Controlled Trial
- Practical Review of Olfactory Training and COVID-19
- Contemporary Review of Olfactory Dysfunction in COVID-19
- The Prevalence of Post-Traumatic Stress Disorder in the General Population during the COVID-19 Pandemic: A Systematic Review and Single-Arm Meta-Analysis
- Pharmacologic therapy of olfaction disroders induced by COVID-19 upper respiratory infection