J Cerebrovasc Endovasc Neurosurg.
2024 Mar;26(1):85-96. 10.7461/jcen.2023.E2022.07.010.
Curative transvenous embolization for congenital multi-hole pial arteriovenous fistula
- Affiliations
-
- 1The University of Kansas School of Medicine, Kansas City, Kansas, USA
- 2Department of Neurological Surgery, University of Kansas, Kansas City, Kansas, USA
- KMID: 2554051
- DOI: http://doi.org/10.7461/jcen.2023.E2022.07.010
Abstract
Objective
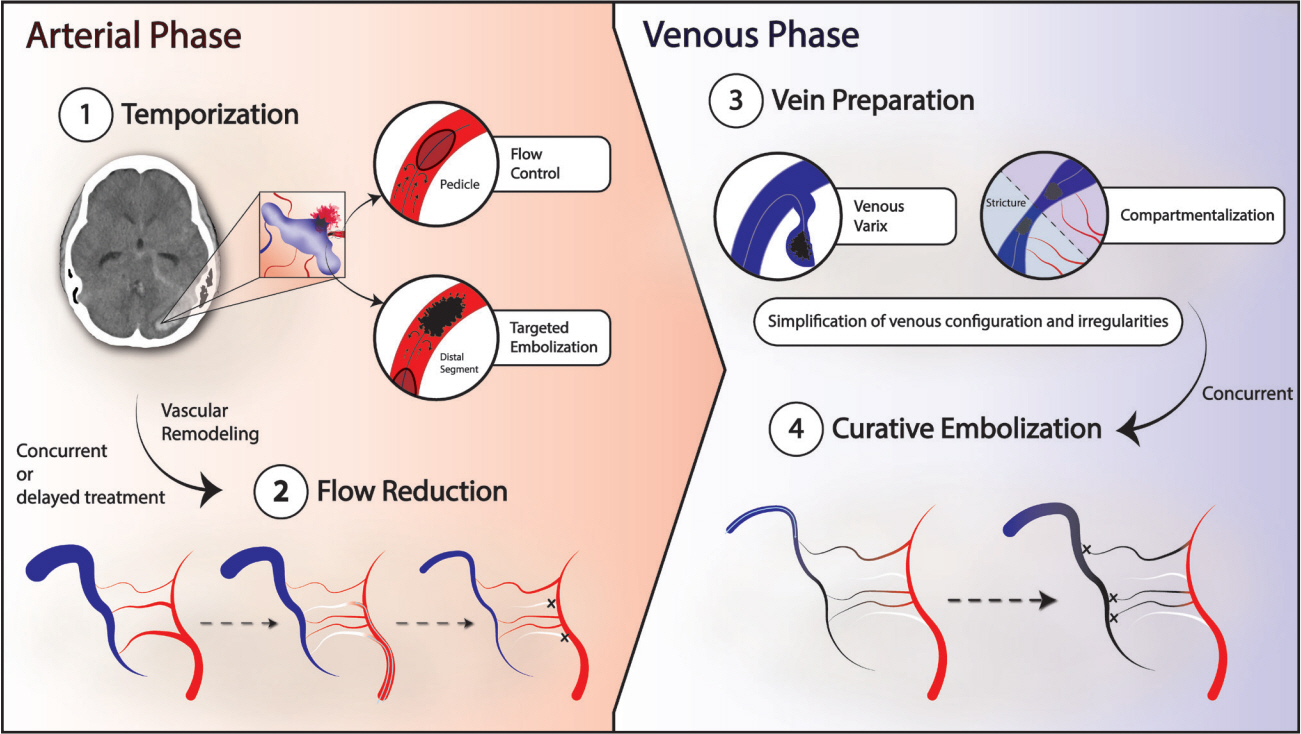
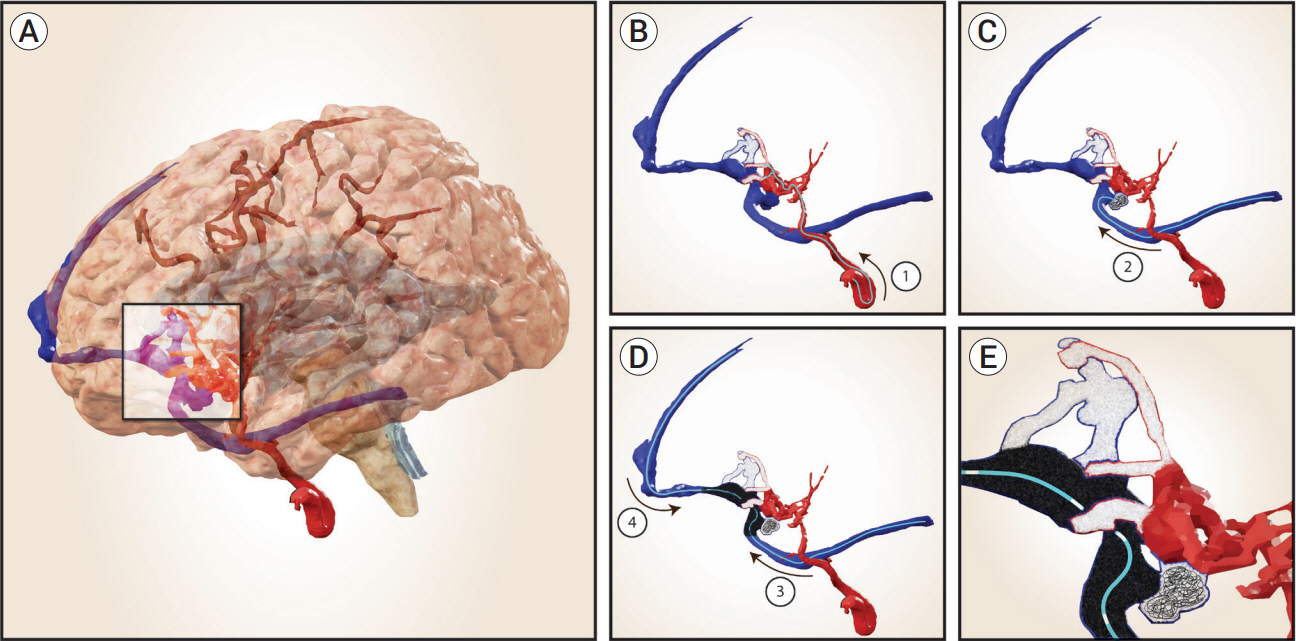
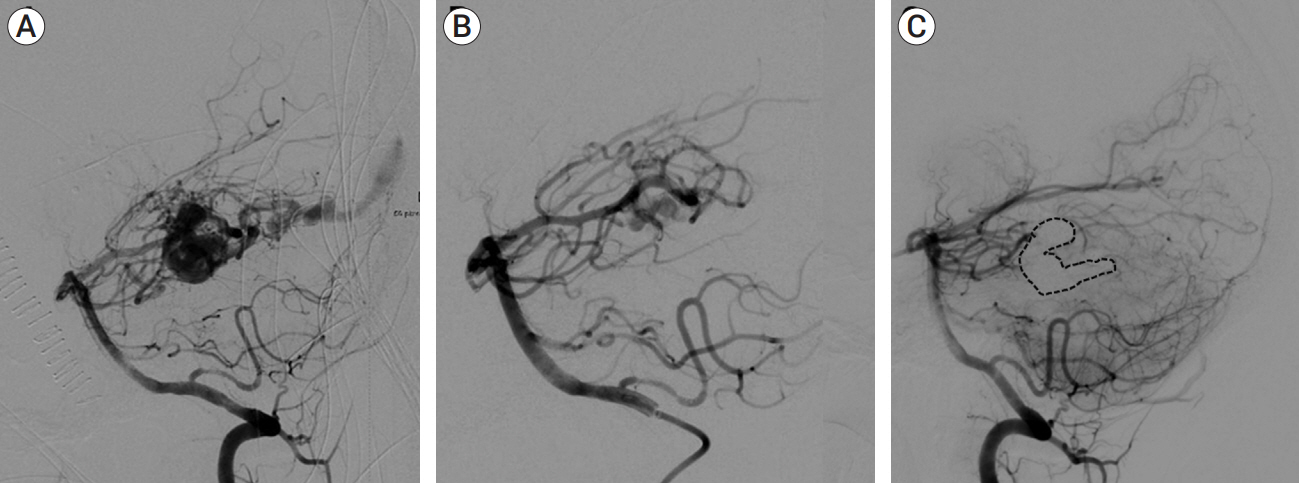
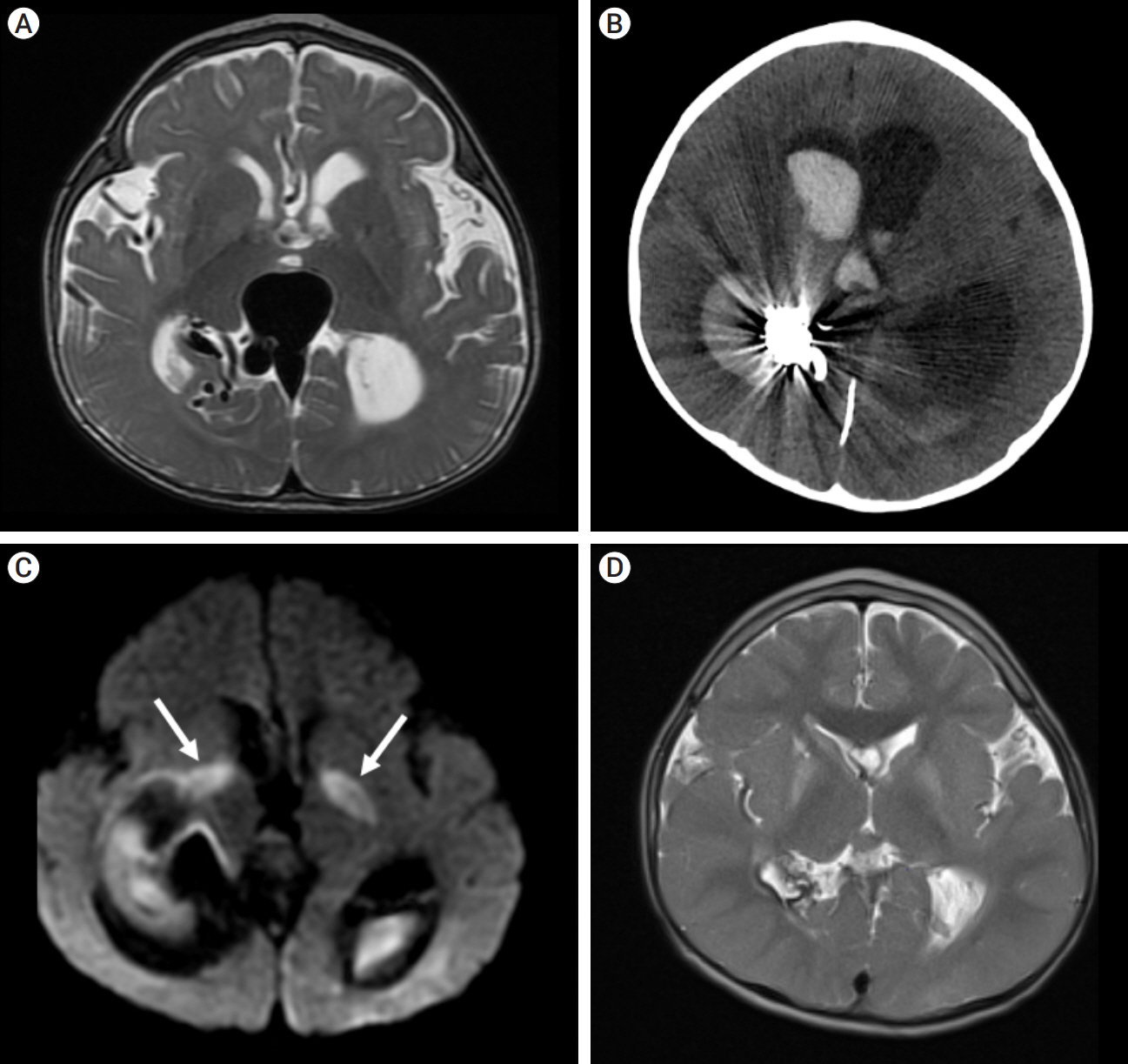
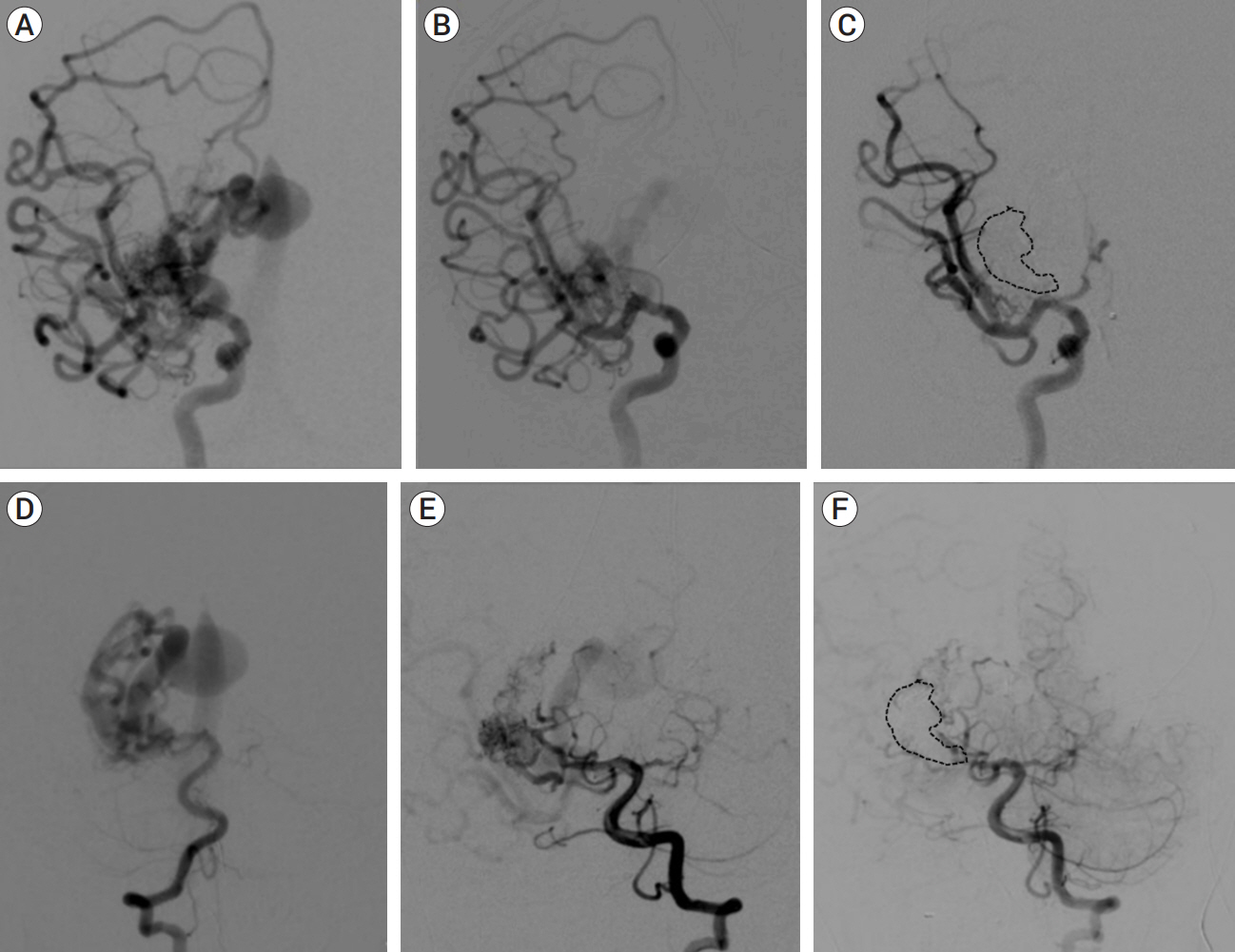
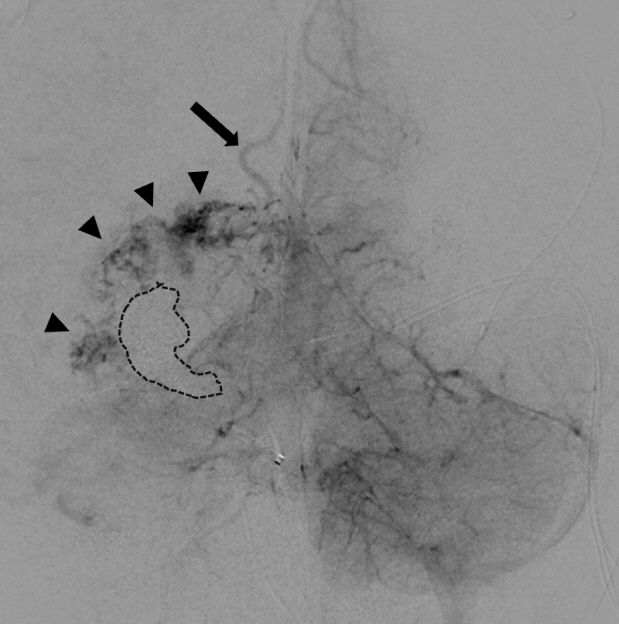
Congenital intracranial pial arteriovenous fistula (PAVF) is a rare cerebral vascular pathology characterized by a direct shunt between one or more pial feeding arteries and a cortical draining vein. Transarterial endovascular embolization (TAE) is widely considered first line therapy. Curative TAE may not be achievable in the multihole variant due to the potential to harbor innumerable small feeding arteries. Transvenous embolization (TVE) may be considered to target the final common outlet of the lesion. Here, we present a series of four patients with complex multi-hole congenital PAVF treated with staged TAE followed by TVE.
Methods
A retrospective review was conducted on patients who underwent treatment for congenital, multi-hole PAVFs treated by a combined TAE/TVE approach at our institution since 2013.
Results
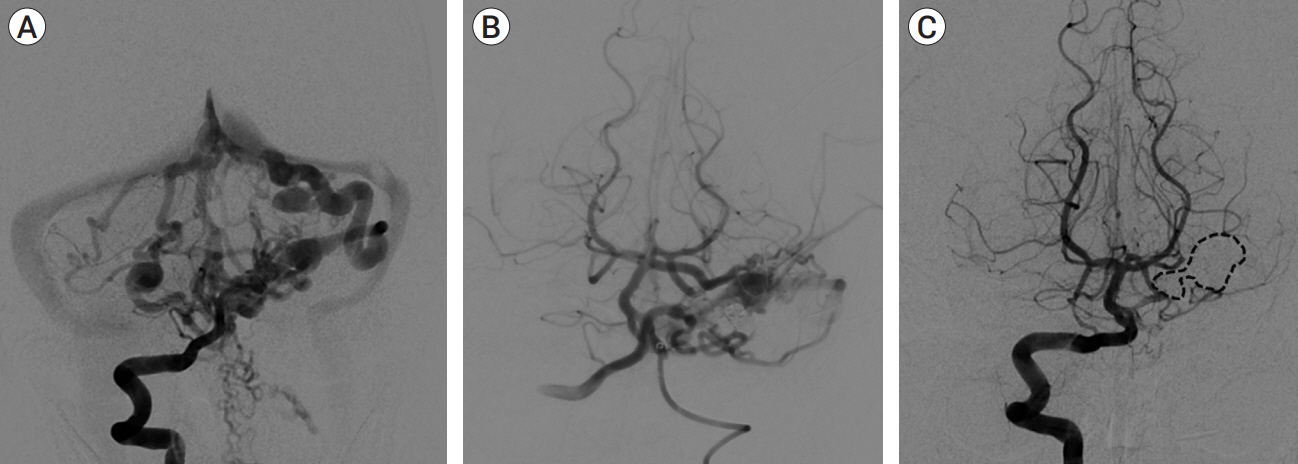
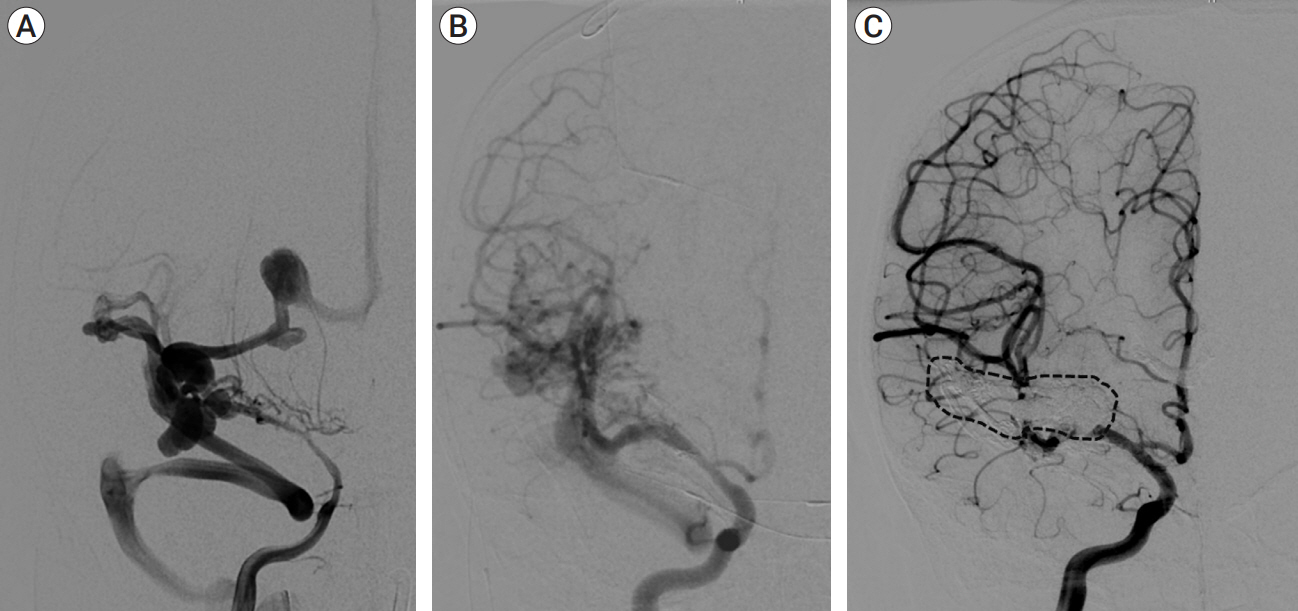
We identified four patients with multi-hole PAVF treated by a combined TAE/TVE. Median age was 5.2 (0-14.7) years. Median follow-up of 8 (1-15) months by catheter angiography and 38 (23-53) months by MRI/MRA was obtained. TVE achieved complete occlusion in three patients that proved durable on radiographic follow-up and demonstrated excellent clinical outcomes with a modified Rankin Score (mRS) of 0 or 1. Complete occlusion of the draining vein was not achieved by TVE in one case. This patient is graded as pediatric mRS=5 three years post-procedure.
Conclusions
With thorough technical considerations, our series indicates that TVE of multi-hole PAVF that are refractory to TAE is feasible and effective in arresting the consequences of chronic, high-flow AV shunting produced by this pathology.
Keyword
Figure
Reference
-
1. Cerebral arteriovenous fistulas. Lasjaunias P, ter Brugge KG, Berenstein A, editors. Surgical Neuroangiography: Clinical and Interventional Aspects in Children. Berlin, Heidelberg: Springer;2006. p. 227–89.2. Cooke D, Tatum J, Farid H, Dowd C, Higashida R, Halbach V. Transvenous embolization of a pediatric pial arteriovenous fistula. J Neurointerv Surg. 2012; Jul. 4(4):e14.
Article3. da Silva Martins WC, de Albuquerque LA, de Souza Filho CB, Dellaretti M, de Sousa AA. Surgical treatment of the intracranial pial arteriovenous fistula. Surg Neurol Int. 2015; Jun. 6:102.
Article4. Eldesouky I. Transvenous endovascular embolisation of nongalenic arteriovenous fistula. EC Neurology. 2015; 1:16–21.5. Goel A, Jain S, Shah A, Rai S, Gore S, Dharurkar P. Pial arteriovenous fistula: A brief review and report of 14 surgically treated cases. World Neurosurg. 2018; Feb. 110:e873–81.
Article6. Guerrero WR, Dandapat S, Ortega-Gutierrez S. Hemorrhagic cerebrovascular pathology in the pediatric population. Front Neurol. 2020; Sep. 11:1055.
Article7. Hetts SW, Keenan K, Fullerton HJ, Young WL, English JD, Gupta N, et al. Pediatric intracranial nongalenic pial arteriovenous fistulas: Clinical features, angioarchitecture, and outcomes. AJNR Am J Neuroradiol. 2012; Oct. 33(9):1710–9.
Article8. Jin H, Meng X, Quan J, Lu Y, Li Y. Role of endovascular embolisation for curative treatment of intracranial non-Galenic pial arteriovenous fistula. Stroke Vasc Neurol. 2021; Jun. 6(2):260–6.
Article9. Li J, Gao Z, Zhi X, Du J, Zhang H, Ling F. Clipping of a pediatric pial arteriovenous fistula located at basilar artery tip using a hybrid trapping-evacuation technique. World Neurosurg. 2018; Sep. 117:292–7.
Article10. Madsen PJ, Lang SS, Pisapia JM, Storm PB, Hurst RW, Heuer GG. An institutional series and literature review of pial arteriovenous fistulas in the pediatric population: Clinical article. J Neurosurg Pediatr. 2013; Oct. 12(4):344–50.11. Maejima R, Ohshima T, Miyachi S, Matsuo N, Kawaguchi R, Takayasu M. Neonatal intracranial pial arteriovenous fistula treated with endovascular embolization: A case report. World Neurosurg. 2018; Oct. 118:261–4.
Article12. Mahmoud M, Abdalla RN, Mohamed AH, Farid M. Pial fistula in infancy: Report of two cases and literature review with special emphasis on the ruptured group. Interv Neuroradiol. 2018; Aug. 24(4):444–9.
Article13. Mao GH, Wang QH. Transvenous embolization with Onyx for a cerebral arteriovenous fistula originating from the distal lenticulostriate artery. Br J Neurosurg. 2013; Aug. 27(4):532–4.
Article14. Medhi G, Gupta AK, Saini J, Ramalingaiah AH, Pendharkar H, Parida S. Pial arteriovenous fistula: A clinical and neuro-interventional experience of outcomes in a rare entity. Indian J Radiol Imaging. 2020; Jul-Sep. 30(3):286–93.
Article15. Okazaki T, Sakamoto S, Ishii D, Oshita J, Matsushige T, Shinagawa K, et al. A pial arteriovenous fistula in infancy as the presenting manifestation of hereditary hemorrhagic telangiectasia. World Neurosurg. 2019; Feb. 122:322–5.
Article16. Orlov K, Gorbatykh A, Berestov V, Shayakhmetov T, Kislitsin D, Seleznev P, et al. Superselective transvenous embolization with Onyx and n-BCA for vein of Galen aneurysmal malformations with restricted transarterial access: Safety, efficacy, and technical aspects. Childs Nerv Syst. 2017; Nov. 33(11):2003–10.
Article17. Requejo F, Jaimovich R, Marelli J, Zuccaro G. Intracranial pial fistulas in pediatric population. Clinical features and treatment modalities. Childs Nerv Syst. 2015; Sep. 31(9):1509–14.
Article18. Terada A, Komiyama M, Ishiguro T, Niimi Y, Oishi H. Nationwide survey of pediatric intracranial arteriovenous shunts in Japan: Japanese Pediatric Arteriovenous Shunts Study (JPAS). J Neurosurg Pediatr. 2018; Nov. 22(5):550–8.
Article19. Tomycz L, Maris AS, Ghiassi M, Singer RJ. Open surgical disconnection for congenital, multi-hole, pial arteriovenous fistulae in non-eloquent cortex. Neurol India. 2012; Jul-Aug. 60(4):415–8.
Article20. Vasudevan K, Spader HS, Grossberg JA, Murphy T, Jayaraman MV. Successful endovascular treatment of a holo-hemispheric cerebral arteriovenous fistula in an infant. J Neurointerv Surg. 2012; Sep. 4(5):e26.
Article21. Viñuela F, Drake CG, Fox AJ, Pelz DM. Giant intracranial varices secondary to high-flow arteriovenous fistulae. J Neurosurg. 1987; Feb. 66(2):198–203.
Article22. Yamada H, Akiyama T, Kamamoto D, Yoshida K, Fukumura M, Toda M. Combined transarterial and transvenous embolization of multi-hole pial arteriovenous fistula with large varix. Neuroradiol J. 2022; Oct. 35(5):640–6.
Article23. Yamashita K, Ohe N, Yoshimura S, Iwama T. Intracranial pial arteriovenous fistula. Neurol Med Chir (Tokyo). 2007; Dec. 47(12):550–4.24. Yang J, Kwon OK, Oh CW, Hwang G, Song KS, Lee YJ. Surgical flow disconnection of cerebral pial dual-channel arteriovenous fistula with a large varix: The role of anti-platelet agent or anti-coagulation therapy. Childs Nerv Syst. 2013; Jun. 29(6):1021–5.
Article25. Yu J, Shi L, Lv X, Wu Z, Yang H. Intracranial non-galenic pial arteriovenous fistula: A review of the literature. Interv Neuroradiol. 2016; Oct. 22(5):557–68.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Successful Treatment of Intracranial Small Pial Single-Channel Arteriovenous Fistula Using N-butyl Cyanoacrylate: Report of 2 Cases
- Congenital Intracranial Pial Arteriovenous Fistula Complicated with Congestive Heart Failure in Neonate: A Case Report
- Coil Embolization of High-flow Pial Arteriovenous Fistula and Management of Hyperperfusion Syndrome: a Case Report
- Embolization of Cerebral Pial Arteriovenous Fistula Under Balloon-assisted Flow Control Using NBCA: a Case Report
- A Case of Congenital Renal Arteriovenous Fistula