J Liver Cancer.
2024 Mar;24(1):17-22. 10.17998/jlc.2023.12.11.
Intrahepatic cholangiocarcinoma: histological diversity and the role of the pathologist
- Affiliations
-
- 1Department of Pathology, International University of Health and Welfare School of Medicine, IUHW Narita Hospital, Chiba, Japan
- KMID: 2553814
- DOI: http://doi.org/10.17998/jlc.2023.12.11
Abstract
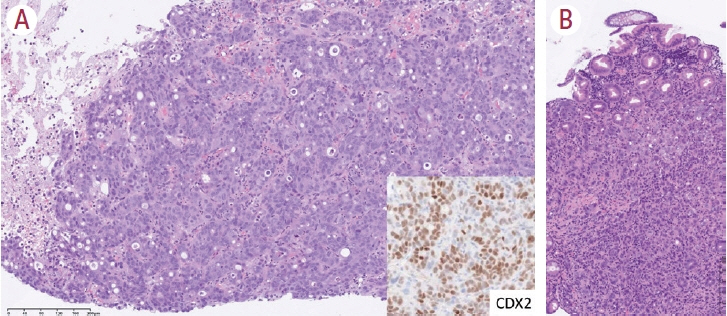
- Intrahepatic cholangiocarcinoma (iCCA) is one of the primary liver cancers and presents with tumor heterogeneity. About 50% of iCCAs comprise actionable mutations, which completely change patient management. In addition, the precise diagnosis of iCCA, including subtype, has become crucial, and pathologists play an important role in this regard. This review focuses on iCCA heterogeneity; looking at different perspectives to guide diagnosis and optimal treatment choice.
Keyword
Figure
Cited by 1 articles
-
Imaging findings of intrahepatic cholangiocarcinoma for prognosis prediction and treatment decision-making: a narrative review
Jun Gu Kang, Taek Chung, Dong Kyu Kim, Hyungjin Rhee
Ewha Med J. 2024;47(4):e66. doi: 10.12771/emj.2024.e66.
Reference
-
References
1. Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020; 17:557–588.
Article2. Komuta M, Ueno A, Sakamoto M. The spectrum of primary liver cancers: heterogeneity and continuity. A foundation for diagnosis and treatment of cancer. Hepatology. 2023; 77:10–12.
Article3. Komuta M. Histological heterogeneity of primary liver cancers: clinical relevance, diagnostic pitfalls and the pathologist’s role. Cancers (Basel). 2021; 13:2871.
Article4. Jusakul A, Cutcutache I, Yong CH, Lim JQ, Huang MN, Padmanabhan N, et al. Whole-Genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discov. 2017; 7:1116–1135.
Article5. Tomczak A, Springfeld C, Dill MT, Chang DH, Kazdal D, Wagner U, et al. Precision oncology for intrahepatic cholangiocarcinoma in clinical practice. Br J Cancer. 2022; 127:1701–1708.
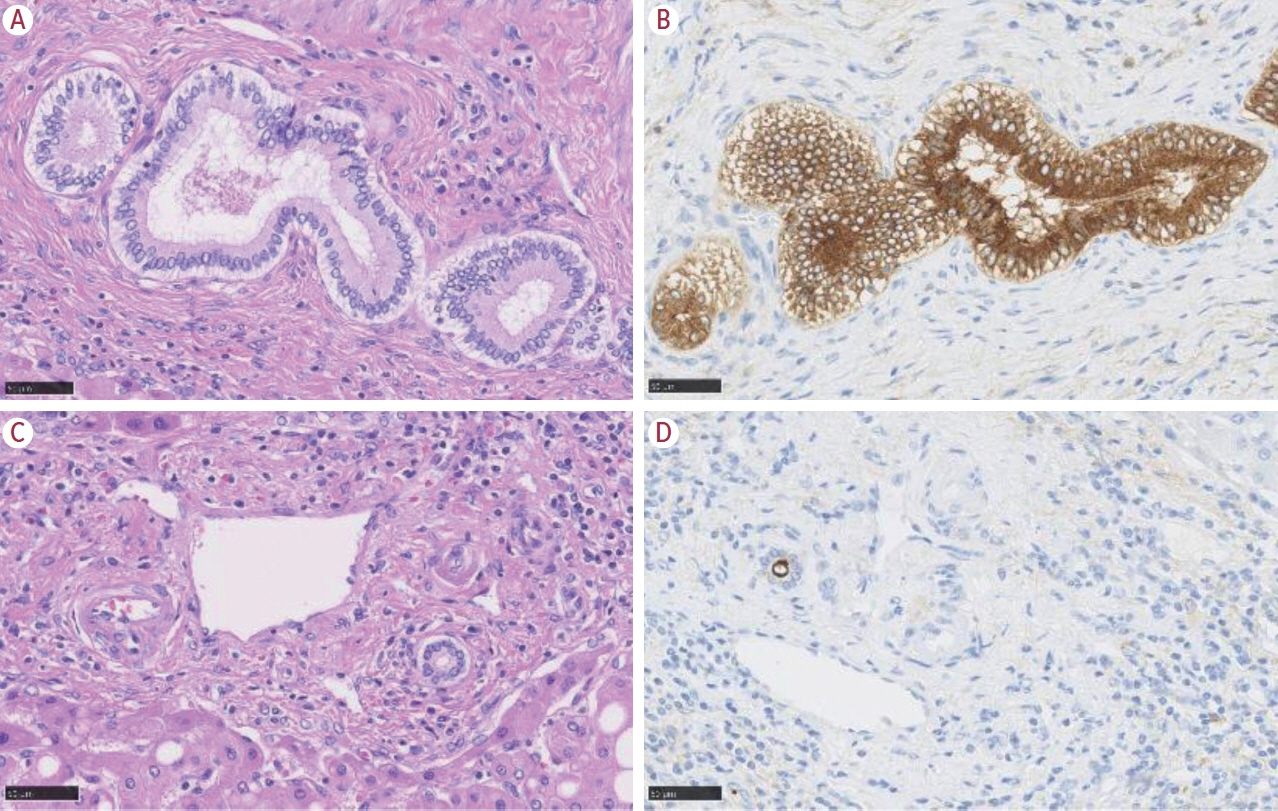
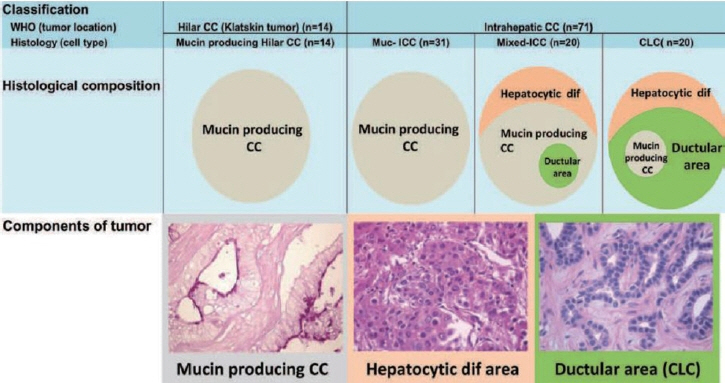
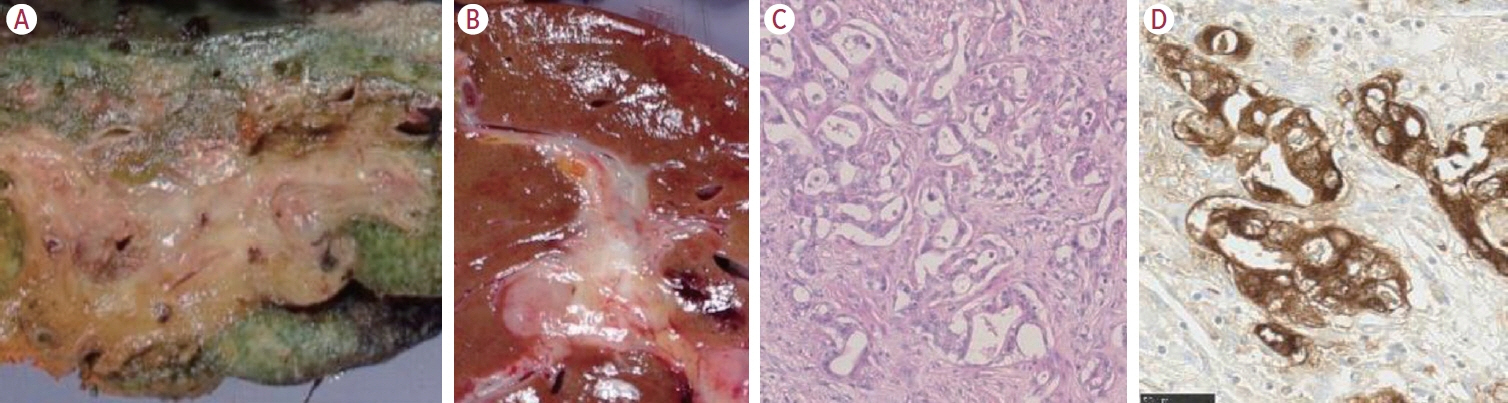
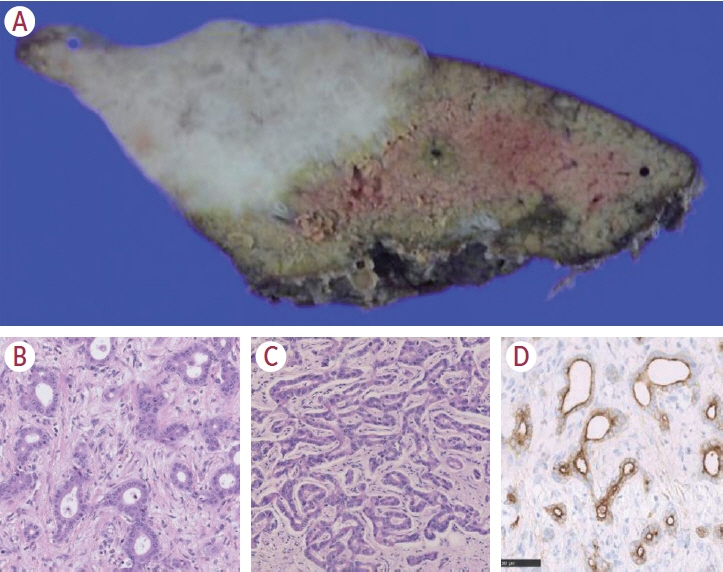
Article6. Nakanuma Y, Klimstra D, Komuta M, Zen Y. Intrahepatic cholangiocarcinoma. 5th ed. Lyon: International Agency for Research on Cancer;2019.7. Komuta M, Govaere O, Vandecaveye V, Akiba J, Van Steenbergen W, Verslype C, et al. Histological diversity in cholangiocellular carcinoma reflects the different cholangiocyte phenotypes. Hepatology. 2012; 55:1876–1888.
Article8. Sasatomi E, Nalesnik MA, Marsh JW. Neuroendocrine carcinoma of the extrahepatic bile duct: case report and literature review. World J Gastroenterol. 2013; 19:4616–4623.
Article9. Kendre G, Murugesan K, Brummer T, Segatto O, Saborowski A, Vogel A. Charting co-mutation patterns associated with actionable drivers in intrahepatic cholangiocarcinoma. J Hepatol. 2023; 78:614–626.
Article10. Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020; 21:671–684.
Article11. Zhu AX, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, et al. Final overall survival efficacy results of ivosidenib for patients with advanced cholangiocarcinoma with IDH1 mutation: the phase 3 randomized clinical ClarIDHy trial. JAMA Oncol. 2021; 7:1669–1677.
Article12. Komuta M. Intrahepatic cholangiocarcinoma: tumour heterogeneity and its clinical relevance. Clin Mol Hepatol. 2022; 28:396–407.
Article13. Hayashi A, Misumi K, Shibahara J, Arita J, Sakamoto Y, Hasegawa K, et al. Distinct clinicopathologic and genetic features of 2 histologic subtypes of intrahepatic cholangiocarcinoma. Am J Surg Pathol. 2016; 40:1021–1030.
Article14. Chung T, Park YN. Up-to-date pathologic classification and molecular characteristics of intrahepatic cholangiocarcinoma. Front Med (Lausanne). 2022; 9:857140.
Article15. Komuta M, Spee B, Borght SV, De Vos R, Verslype C, Aerts R, et al. Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. Hepatology. 2008; 47:1544–1556.
Article16. Yan BC, Gong C, Song J, Krausz T, Tretiakova M, Hyjek E, et al. Arginase-1: a new immunohistochemical marker of hepatocytes and hepatocellular neoplasms. Am J Surg Pathol. 2010; 34:1147–1154.17. Komuta M, Yeh MM. A review on the update of combined hepatocellular cholangiocarcinoma. Semin Liver Dis. 2020; 40:124–130.
Article18. Gigante E, Ronot M, Bertin C, Ciolina M, Bouattour M, Dondero F, et al. Combining imaging and tumour biopsy improves the diagnosis of combined hepatocellular-cholangiocarcinoma. Liver Int. 2019; 39:2386–2396.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Histopathology of a benign bile duct lesion in the liver: Morphologic mimicker or precursor of intrahepatic cholangiocarcinoma
- Recent Updates in the Imaging Diagnosis of Cholangiocarcinoma
- Intrahepatic Cholangiocarcinoma: Evolving Concepts and Medical Treatment
- Intrahepatic cholangiocarcinoma associated with Clonorchis sinensis infection
- A Case of Intrahepatic Cholangiocarcinoma Developed in a Remote Region from the Site of Hepatolithiasis