Diabetes Metab J.
2024 Mar;48(2):215-230. 10.4093/dmj.2022.0332.
Extracellular Vimentin Alters Energy Metabolism And Induces Adipocyte Hypertrophy
- Affiliations
-
- 1Department of Medicine, Graduate School, Ewha Womans University, Seoul, Korea
- 2Department of Molecular Medicine, Ewha Womans University College of Medicine, Seoul, Korea
- KMID: 2553593
- DOI: http://doi.org/10.4093/dmj.2022.0332
Abstract
- Background
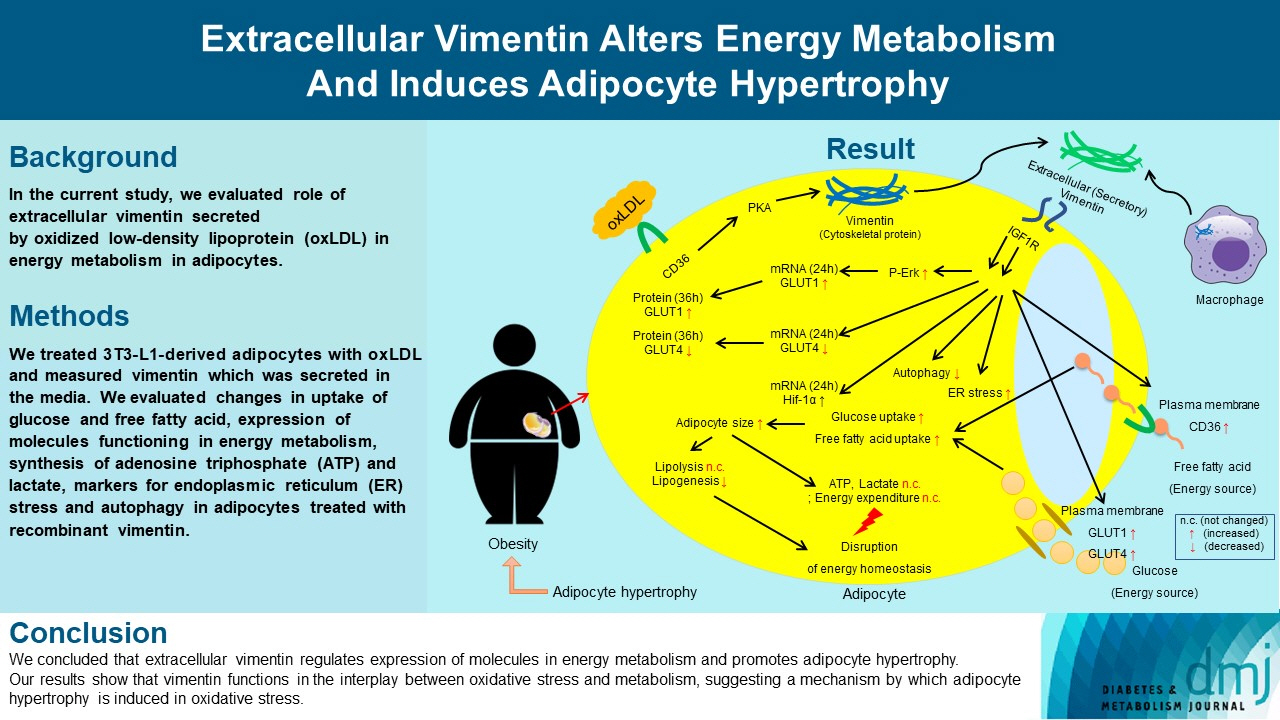
Previous studies have reported that oxidative stress contributes to obesity characterized by adipocyte hypertrophy. However, mechanism has not been studied extensively. In the current study, we evaluated role of extracellular vimentin secreted by oxidized low-density lipoprotein (oxLDL) in energy metabolism in adipocytes.
Methods
We treated 3T3-L1-derived adipocytes with oxLDL and measured vimentin which was secreted in the media. We evaluated changes in uptake of glucose and free fatty acid, expression of molecules functioning in energy metabolism, synthesis of adenosine triphosphate (ATP) and lactate, markers for endoplasmic reticulum (ER) stress and autophagy in adipocytes treated with recombinant vimentin.
Results
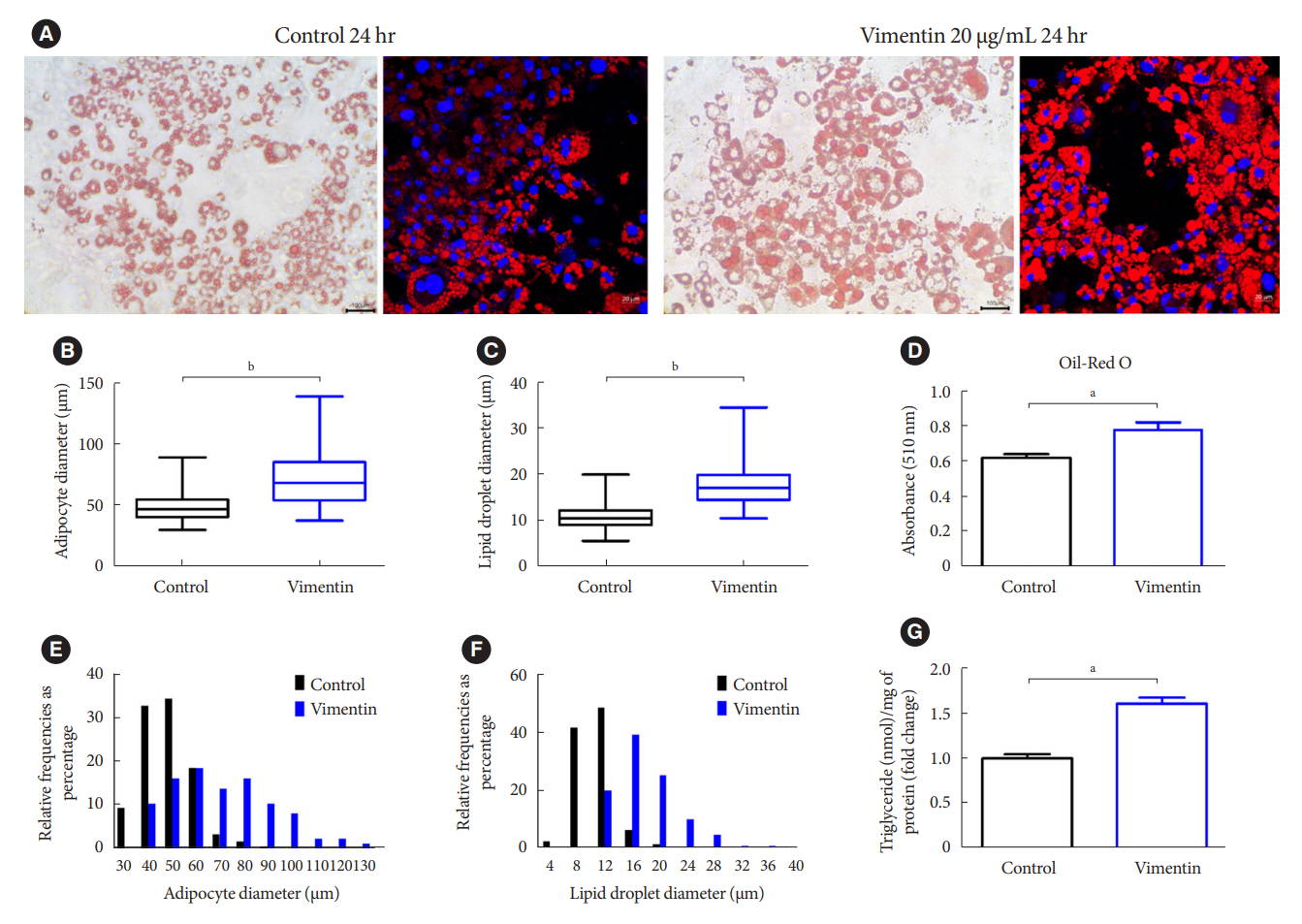
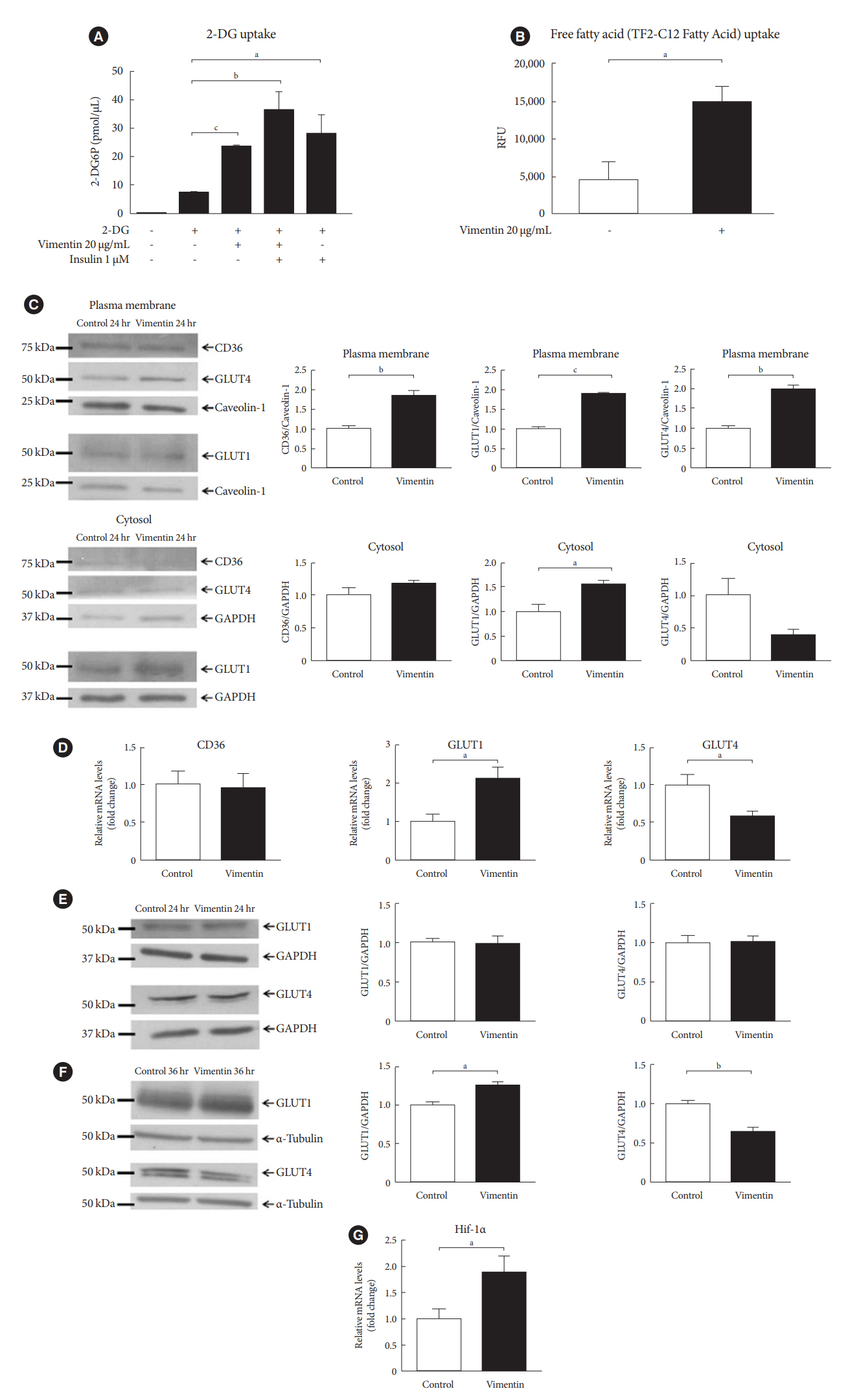
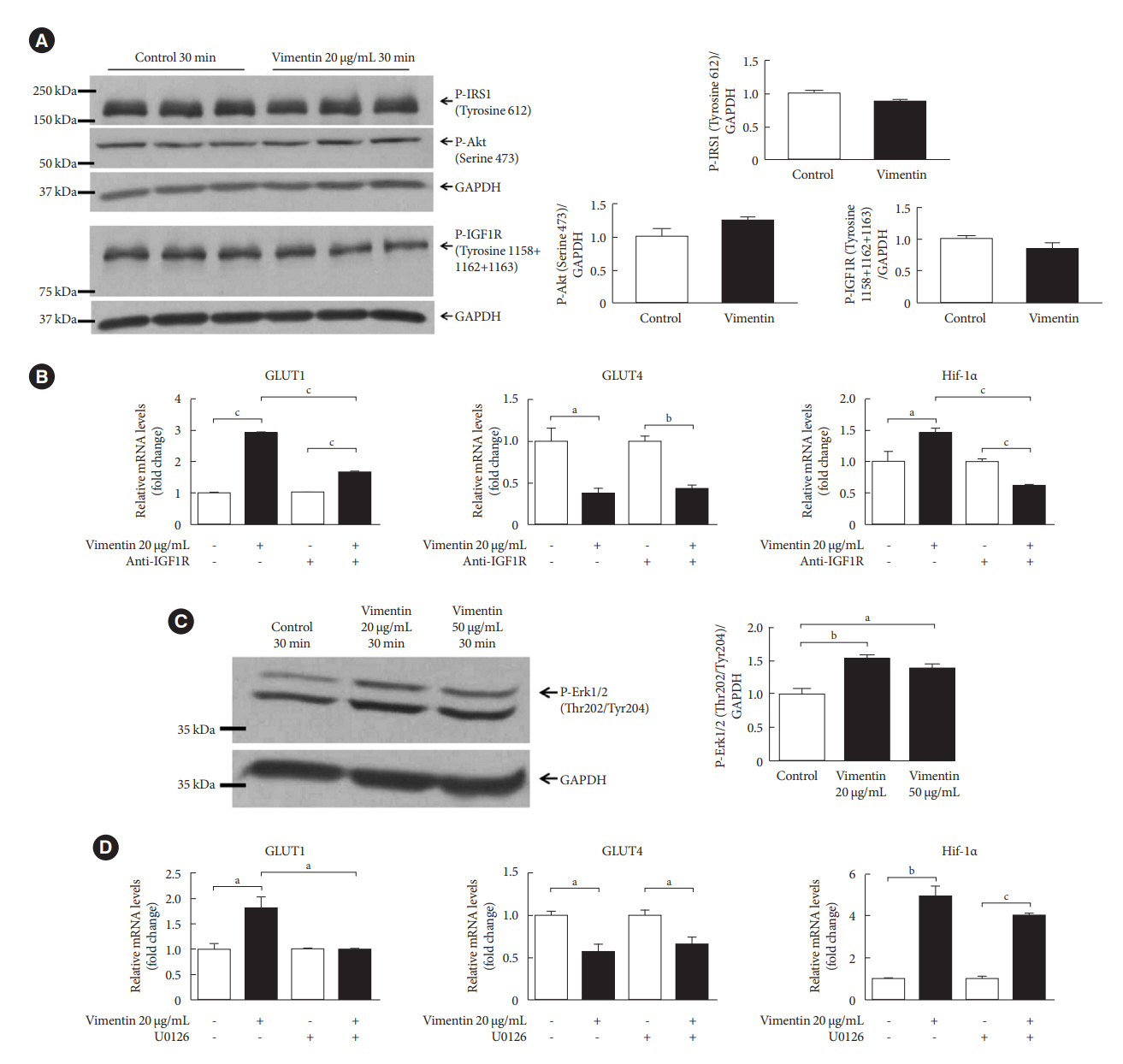
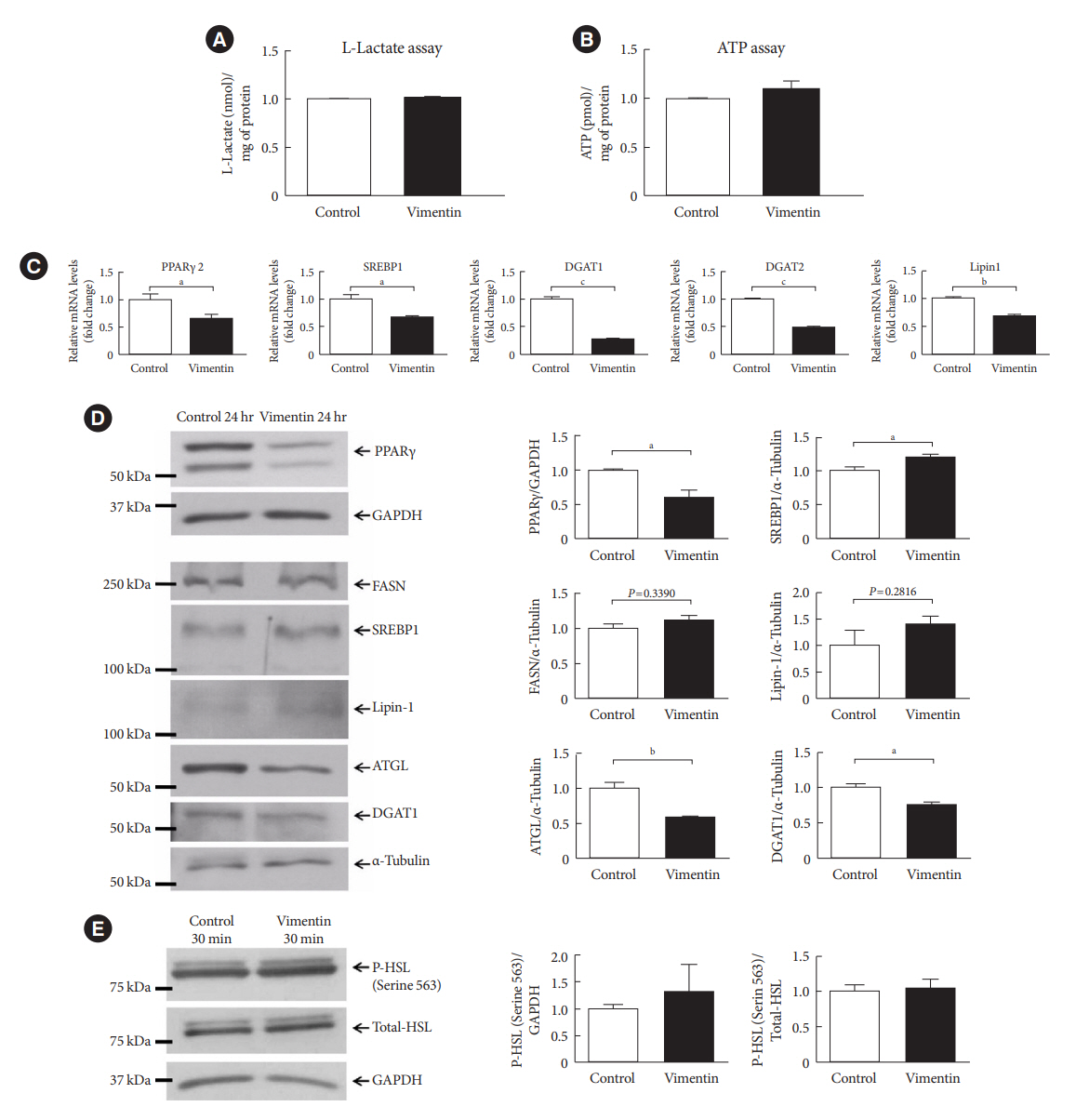
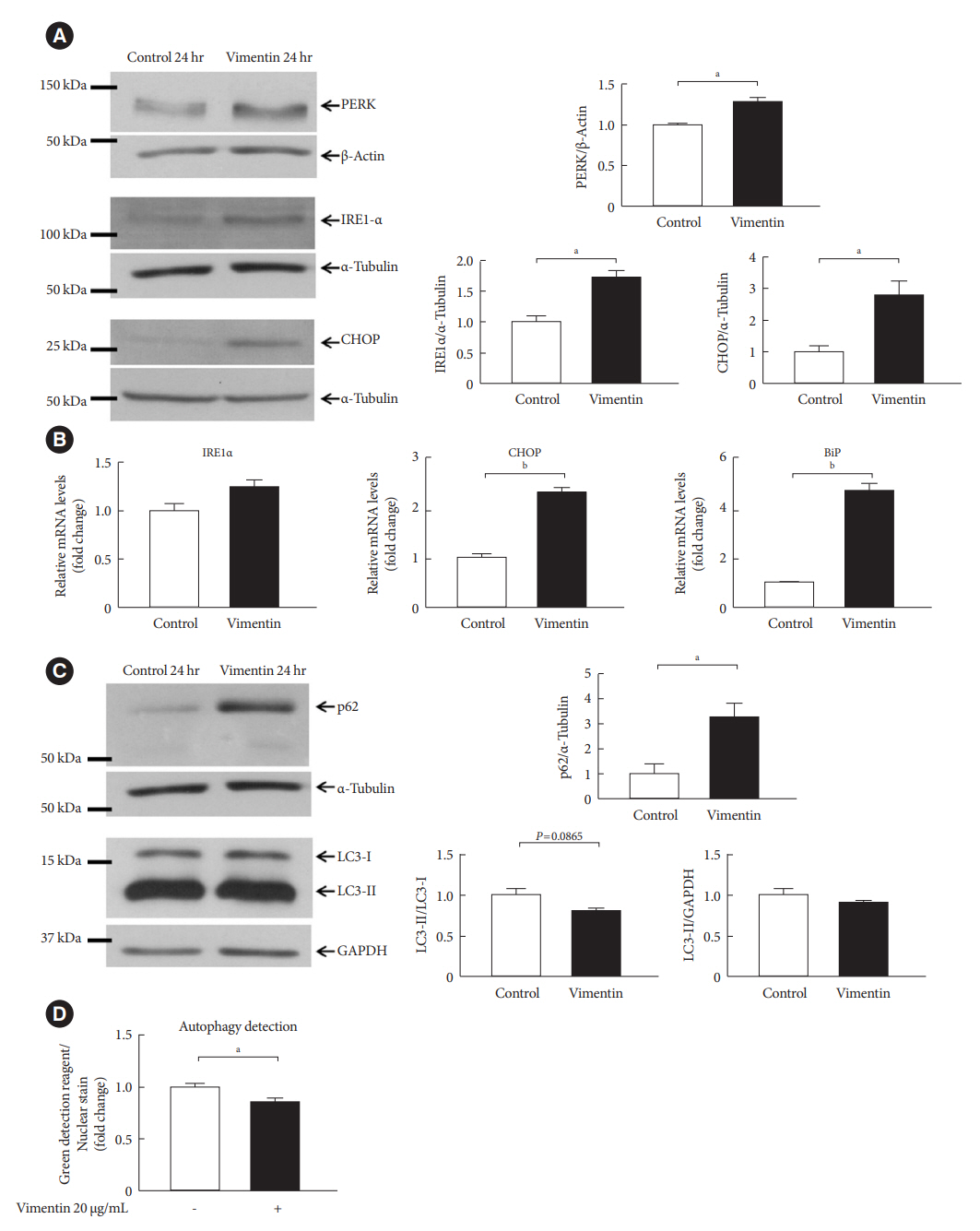
Adipocytes secreted vimentin in response to oxLDL. Microscopic evaluation revealed that vimentin treatment induced increase in adipocyte size and increase in sizes of intracellular lipid droplets with increased intracellular triglyceride. Adipocytes treated with vimentin showed increased uptake of glucose and free fatty acid with increased expression of plasma membrane glucose transporter type 1 (GLUT1), GLUT4, and CD36. Vimentin treatment increased transcription of GLUT1 and hypoxia-inducible factor 1α (Hif-1α) but decreased GLUT4 transcription. Adipose triglyceride lipase (ATGL), peroxisome proliferator-activated receptor γ (PPARγ), sterol regulatory element-binding protein 1 (SREBP1), diacylglycerol O-acyltransferase 1 (DGAT1) and 2 were decreased by vimentin treatment. Markers for ER stress were increased and autophagy was impaired in vimentin-treated adipocytes. No change was observed in synthesis of ATP and lactate in the adipocytes treated with vimentin.
Conclusion
We concluded that extracellular vimentin regulates expression of molecules in energy metabolism and promotes adipocyte hypertrophy. Our results show that vimentin functions in the interplay between oxidative stress and metabolism, suggesting a mechanism by which adipocyte hypertrophy is induced in oxidative stress.
Keyword
Figure
Reference
-
1. Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. 2004; 89:2595–600.
Article2. Garcia-Sanchez A, Gamez-Nava JI, Diaz-de la Cruz EN, Cardona-Munoz EG, Becerra-Alvarado IN, Aceves-Aceves JA, et al. The effect of visceral abdominal fat volume on oxidative stress and proinflammatory cytokines in subjects with normal weight, overweight and obesity. Diabetes Metab Syndr Obes. 2020; 13:1077–87.3. Pennathur S, Heinecke JW. Mechanisms of oxidative stress in diabetes: implications for the pathogenesis of vascular disease and antioxidant therapy. Front Biosci. 2004; 9:565–74.
Article4. Lee H, Lee YJ, Choi H, Ko EH, Kim JW. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J Biol Chem. 2009; 284:10601–9.
Article5. Njajou OT, Kanaya AM, Holvoet P, Connelly S, Strotmeyer ES, Harris TB, et al. Association between oxidized LDL, obesity and type 2 diabetes in a population-based cohort, the Health, Aging and Body Composition Study. Diabetes Metab Res Rev. 2009; 25:733–9.
Article6. Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci. 2013; 9:191–200.7. Kawai T, Autieri MV, Scalia R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am J Physiol Cell Physiol. 2021; 320:C375–91.
Article8. Muir LA, Neeley CK, Meyer KA, Baker NA, Brosius AM, Washabaugh AR, et al. Adipose tissue fibrosis, hypertrophy, and hyperplasia: correlations with diabetes in human obesity. Obesity (Silver Spring). 2016; 24:597–605.
Article9. Heid H, Rickelt S, Zimbelmann R, Winter S, Schumacher H, Dorflinger Y, et al. On the formation of lipid droplets in human adipocytes: the organization of the perilipin-vimentin cortex. PLoS One. 2014; 9:e90386.
Article10. Kim S, Kim I, Cho W, Oh GT, Park YM. Vimentin deficiency prevents high-fat diet-induced obesity and insulin resistance in mice. Diabetes Metab J. 2021; 45:97–108.
Article11. Mor-Vaknin N, Punturieri A, Sitwala K, Markovitz DM. Vimentin is secreted by activated macrophages. Nat Cell Biol. 2003; 5:59–63.
Article12. Kim S, Cho W, Kim I, Lee SH, Oh GT, Park YM. Oxidized LDL induces vimentin secretion by macrophages and contributes to atherosclerotic inflammation. J Mol Med (Berl). 2020; 98:973–83.
Article13. Gong DH, Dai Y, Chen S, Wang XQ, Yan XX, Shen Y, et al. Secretory vimentin is associated with coronary artery disease in patients and induces atherogenesis in ApoE-/- mice. Int J Cardiol. 2019; 283:9–16.
Article14. Jay AG, Chen AN, Paz MA, Hung JP, Hamilton JA. CD36 binds oxidized low density lipoprotein (LDL) in a mechanism dependent upon fatty acid binding. J Biol Chem. 2015; 290:4590–603.
Article15. Eriksson JE, He T, Trejo-Skalli AV, Harmala-Brasken AS, Hellman J, Chou YH, et al. Specific in vivo phosphorylation sites determine the assembly dynamics of vimentin intermediate filaments. J Cell Sci. 2004; 117(Pt 6):919–32.
Article16. Kim SY, Jeong SJ, Park JH, Cho W, Ahn YH, Choi YH, et al. Plasma membrane localization of CD36 requires vimentin phosphorylation; a mechanism by which macrophage vimentin promotes atherosclerosis. Front Cardiovasc Med. 2022; 9:792717.
Article17. Rea S, James DE. Moving GLUT4: the biogenesis and trafficking of GLUT4 storage vesicles. Diabetes. 1997; 46:1667–77.
Article18. Wieman HL, Wofford JA, Rathmell JC. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol Biol Cell. 2007; 18:1437–46.
Article19. Shigyo M, Tohda C. Extracellular vimentin is a novel axonal growth facilitator for functional recovery in spinal cord-injured mice. Sci Rep. 2016; 6:28293.
Article20. Li W, Miller WT. Role of the activation loop tyrosines in regulation of the insulin-like growth factor I receptor-tyrosine kinase. J Biol Chem. 2006; 281:23785–91.
Article21. Montessuit C, Thorburn A. Transcriptional activation of the glucose transporter GLUT1 in ventricular cardiac myocytes by hypertrophic agonists. J Biol Chem. 1999; 274:9006–12.
Article22. Basse AL, Isidor MS, Winther S, Skjoldborg NB, Murholm M, Andersen ES, et al. Regulation of glycolysis in brown adipocytes by HIF-1α. Sci Rep. 2017; 7:4052.
Article23. Reue K, Dwyer JR. Lipin proteins and metabolic homeostasis. J Lipid Res. 2009; 50 Suppl:S109–14.
Article24. Zechner R, Zimmermann R, Eichmann TO, Kohlwein SD, Haemmerle G, Lass A, et al. FAT SIGNALS: lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012; 15:279–91.25. Koumenis C, Naczki C, Koritzinsky M, Rastani S, Diehl A, Sonenberg N, et al. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2alpha. Mol Cell Biol. 2002; 22:7405–16.26. Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006; 313:1137–40.
Article27. Shu S, Wang H, Zhu J, Liu Z, Yang D, Wu W, et al. Reciprocal regulation between ER stress and autophagy in renal tubular fibrosis and apoptosis. Cell Death Dis. 2021; 12:1016.
Article28. Perez-Sala D, Oeste CL, Martinez AE, Carrasco MJ, Garzon B, Canada FJ. Vimentin filament organization and stress sensing depend on its single cysteine residue and zinc binding. Nat Commun. 2015; 6:7287.
Article29. Rhoads JP, Major AS. How oxidized low-density lipoprotein activates inflammatory responses. Crit Rev Immunol. 2018; 38:333–42.
Article30. Santiago-Fernandez C, Martin-Reyes F, Tome M, Ocana-Wilhelmi L, Rivas-Becerra J, Tatzber F, et al. Oxidized LDL modify the human adipocyte phenotype to an insulin resistant, proinflamatory and proapoptotic profile. Biomolecules. 2020; 10:534.
Article31. Ebeling P, Koistinen HA, Koivisto VA. Insulin-independent glucose transport regulates insulin sensitivity. FEBS Lett. 1998; 436:301–3.
Article32. Hwang DY, Ismail-Beigi F. Stimulation of GLUT-1 glucose transporter expression in response to hyperosmolarity. Am J Physiol Cell Physiol. 2001; 281:C1365–72.
Article33. Beg M, Abdullah N, Thowfeik FS, Altorki NK, McGraw TE. Distinct Akt phosphorylation states are required for insulin regulated Glut4 and Glut1-mediated glucose uptake. Elife. 2017; 6:e26896.
Article34. Feng J, Han J, Pearce SF, Silverstein RL, Gotto AM Jr, Hajjar DP, et al. Induction of CD36 expression by oxidized LDL and IL-4 by a common signaling pathway dependent on protein kinase C and PPAR-gamma. J Lipid Res. 2000; 41:688–96.35. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993; 259:87–91.
Article36. McGowan KM, Police S, Winslow JB, Pekala PH. Tumor necrosis factor-alpha regulation of glucose transporter (GLUT1) mRNA turnover: contribution of the 3’-untranslated region of the GLUT1 message. J Biol Chem. 1997; 272:1331–7.37. Cornelius P, Marlowe M, Lee MD, Pekala PH. The growth factor-like effects of tumor necrosis factor-alpha. Stimulation of glucose transport activity and induction of glucose transporter and immediate early gene expression in 3T3-L1 preadipocytes. J Biol Chem. 1990; 265:20506–16.
Article38. Bird TA, Davies A, Baldwin SA, Saklatvala J. Interleukin 1 stimulates hexose transport in fibroblasts by increasing the expression of glucose transporters. J Biol Chem. 1990; 265:13578–83.
Article39. Pendergrass M, Koval J, Vogt C, Yki-Jarvinen H, Iozzo P, Pipek R, et al. Insulin-induced hexokinase II expression is reduced in obesity and NIDDM. Diabetes. 1998; 47:387–94.
Article40. Laufs U, Parhofer KG, Ginsberg HN, Hegele RA. Clinical review on triglycerides. Eur Heart J. 2020; 41:99–109. c.
Article41. Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes. 2011; 60:2441–9.
Article42. Baldini F, Fabbri R, Eberhagen C, Voci A, Portincasa P, Zischka H, et al. Adipocyte hypertrophy parallels alterations of mitochondrial status in a cell model for adipose tissue dysfunction in obesity. Life Sci. 2021; 265:118812.
Article43. He Q, Gao Z, Yin J, Zhang J, Yun Z, Ye J. Regulation of HIF-1 {alpha} activity in adipose tissue by obesity-associated factors: adipogenesis, insulin, and hypoxia. Am J Physiol Endocrinol Metab. 2011; 300:E877–85.44. Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006; 3:177–85.
Article45. Kierans SJ, Taylor CT. Regulation of glycolysis by the hypoxiainducible factor (HIF): implications for cellular physiology. J Physiol. 2021; 599:23–37.
Article46. Chen C, Pore N, Behrooz A, Ismail-Beigi F, Maity A. Regulation of glut1 mRNA by hypoxia-inducible factor-1: interaction between H-ras and hypoxia. J Biol Chem. 2001; 276:9519–25.47. Zheng Z, Zhang C, Zhang K. Role of unfolded protein response in lipogenesis. World J Hepatol. 2010; 2:203–7.
Article48. Takahashi N, Yoshizaki T, Hiranaka N, Suzuki T, Yui T, Akanuma M, et al. Endoplasmic reticulum stress suppresses lipin-1 expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2013; 431:25–30.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adipocyte Signals in Energy Balance and Digestive Diseases
- The Role of Vitamin D in Adipose Tissue Biology: Adipocyte Differentiation, Energy Metabolism, and Inflammation
- Brown Fat and Browning for the Treatment of Obesity and Related Metabolic Disorders
- Transcriptional repression of type I procollagen genes during adipocyte differentiation
- The Mechanism of White and Brown Adipocyte Differentiation