Diabetes Metab J.
2024 Mar;48(2):161-169. 10.4093/dmj.2023.0240.
Hepatic Fibrosis and Cancer: The Silent Threats of Metabolic Syndrome
- Affiliations
-
- 1Division of Liver Diseases, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, USA
- KMID: 2553587
- DOI: http://doi.org/10.4093/dmj.2023.0240
Abstract
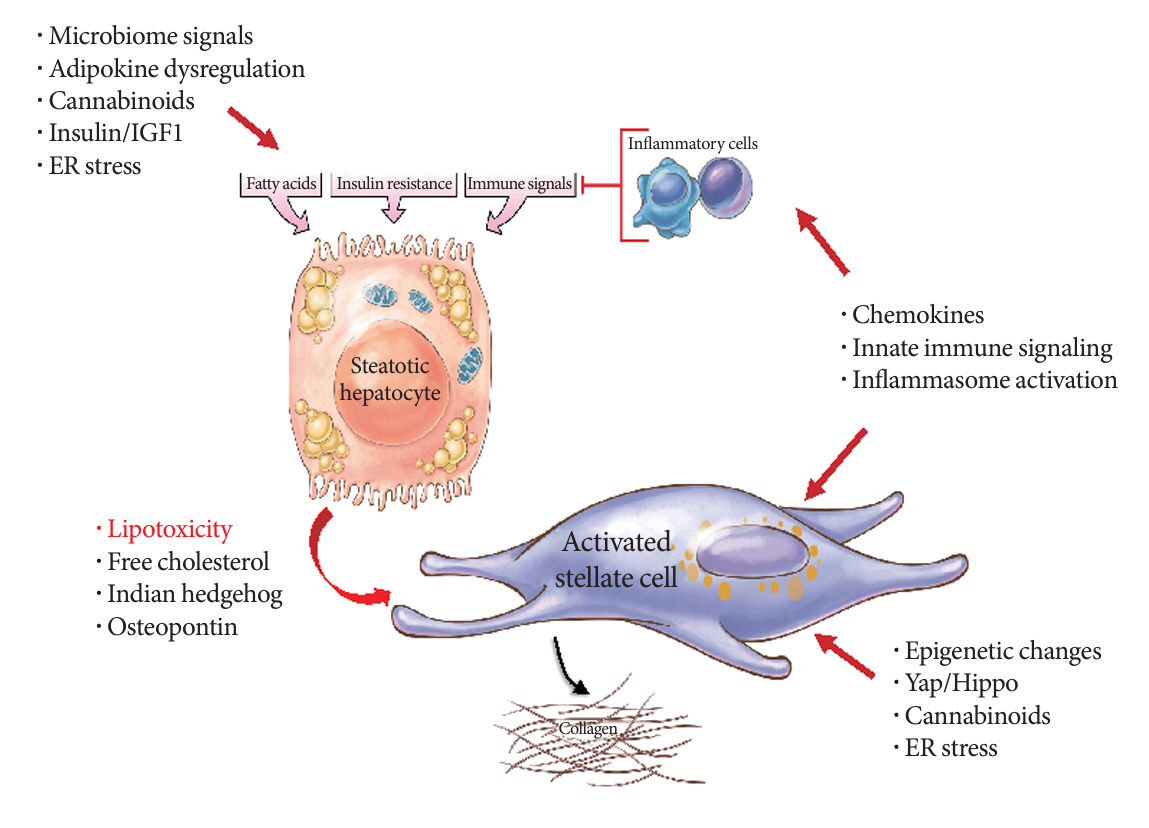
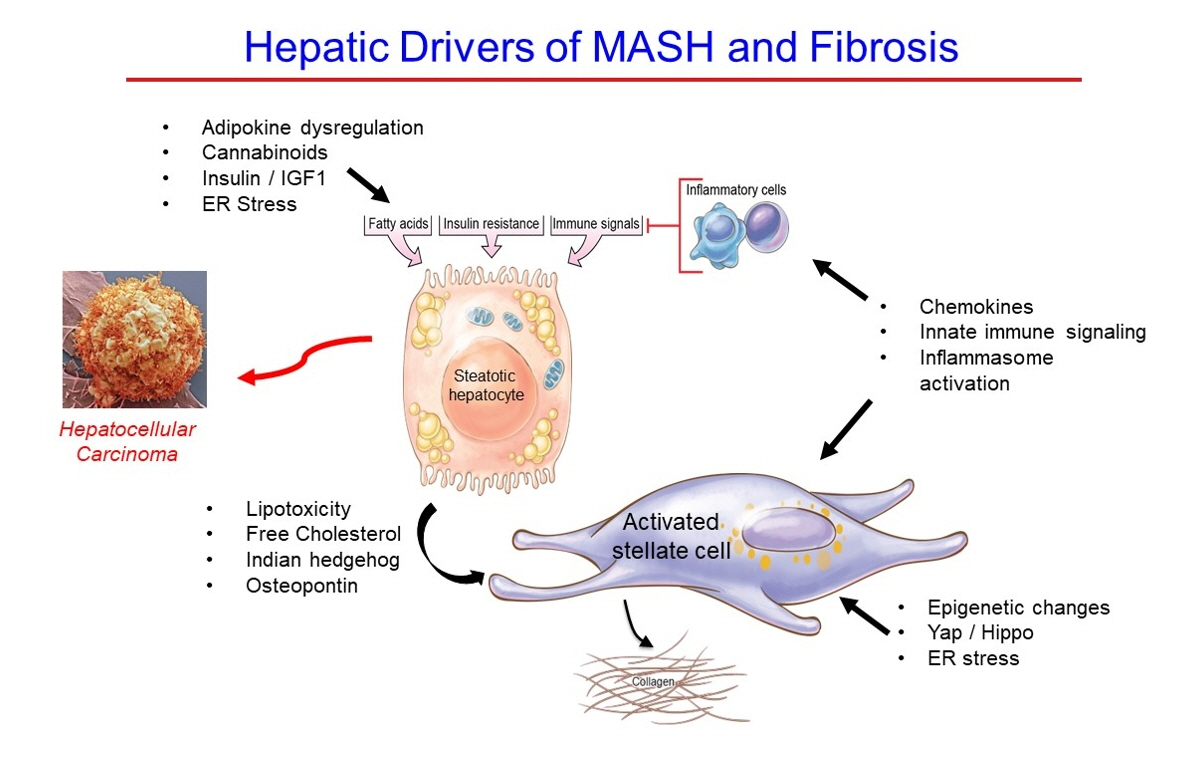
- Metabolic dysfunction-associated steatotic (fatty) liver disease (MASLD), previously termed non-alcoholic fatty liver disease, is a worldwide epidemic that can lead to hepatic inflammation, fibrosis, cirrhosis, and hepatocellular carcinoma (HCC). The disease is typically a component of the metabolic syndrome that accompanies obesity, and is often overlooked because the liver manifestations are clinically silent until late-stage disease is present (i.e., cirrhosis). Moreover, Asian populations, including Koreans, have a higher fraction of patients who are lean, yet their illness has the same prognosis or worse than those who are obese. Nonetheless, ongoing injury can lead to hepatic inflammation and ballooning of hepatocytes as classic features. Over time, fibrosis develops following activation of hepatic stellate cells, the liver’s main fibrogenic cell type. The disease is usually more advanced in patients with type 2 diabetes mellitus, indicating that all diabetic patients should be screened for liver disease. Although there has been substantial progress in clarifying pathways of injury and fibrosis, there no approved therapies yet, but current research seeks to uncover the pathways driving hepatic inflammation and fibrosis, in hopes of identifying new therapeutic targets. Emerging molecular methods, especially single cell sequencing technologies, are revolutionizing our ability to clarify mechanisms underlying MASLD-associated fibrosis and HCC.
Keyword
Figure
Reference
-
1. Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, et al. Endotext. South Dartmouth: MDText.com, Inc;2000. Chapter, Metabolic syndrome [cited 2023 Oct 27]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK278936.2. Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023; 78:1966–86.3. Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021; 18:223–38.
Article4. Loomba R, Lim JK, Patton H, El-Serag HB. AGA clinical practice update on screening and surveillance for hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: expert review. Gastroenterology. 2020; 158:1822–30.
Article5. Wong MC, Huang JL, George J, Huang J, Leung C, Eslam M, et al. The changing epidemiology of liver diseases in the Asia-Pacific region. Nat Rev Gastroenterol Hepatol. 2019; 16:57–73.
Article6. Im HJ, Ahn YC, Wang JH, Lee MM, Son CG. Systematic review on the prevalence of nonalcoholic fatty liver disease in South Korea. Clin Res Hepatol Gastroenterol. 2021; 45:101526.
Article7. Huh Y, Cho YJ, Nam GE. Recent epidemiology and risk factors of nonalcoholic fatty liver disease. J Obes Metab Syndr. 2022; 31:17–27.
Article8. Han E, Han KD, Lee YH, Kim KS, Hong S, Park JH, et al. Fatty liver & diabetes statistics in Korea: nationwide data 2009 to 2017. Diabetes Metab J. 2023; 47:347–55.
Article9. Nah EH, Cho S, Park H, Noh D, Kwon E, Cho HI. Subclinical steatohepatitis and advanced liver fibrosis in health examinees with nonalcoholic fatty liver disease (NAFLD) in 10 South Korean cities: a retrospective cross-sectional study. PLoS One. 2021; 16:e0260477.
Article10. Young S, Tariq R, Provenza J, Satapathy SK, Faisal K, Choudhry A, et al. Prevalence and profile of nonalcoholic fatty liver disease in lean adults: systematic review and meta-analysis. Hepatol Commun. 2020; 4:953–72.
Article11. Ha J, Yim SY, Karagozian R. Mortality and liver-related events in lean versus non-lean nonalcoholic fatty liver disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2023; 21:2496–507.
Article12. Nabi O, Lapidus N, Boursier J, de Ledinghen V, Petit JM, Kab S, et al. Lean individuals with NAFLD have more severe liver disease and poorer clinical outcomes (NASH-CO Study). Hepatology. 2023; 78:272–83.
Article13. Eslam M, Chen F, George J. NAFLD in lean Asians. Clin Liver Dis (Hoboken). 2021; 16:240–3.
Article14. Lazarus JV, Ekstedt M, Marchesini G, Mullen J, Novak K, Pericas JM, et al. A cross-sectional study of the public health response to non-alcoholic fatty liver disease in Europe. J Hepatol. 2020; 72:14–24.
Article15. Lazarus JV, Mark HE, Villota-Rivas M, Palayew A, Carrieri P, Colombo M, et al. The global NAFLD policy review and preparedness index: are countries ready to address this silent public health challenge? J Hepatol. 2022; 76:771–80.16. Younossi ZM, Stepanova M, Lawitz EJ, Reddy KR, Wai-Sun Wong V, Mangia A, et al. Patients with nonalcoholic steatohepatitis experience severe impairment of health-related quality of life. Am J Gastroenterol. 2019; 114:1636–41.
Article17. Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021; 184:2537–64.
Article18. Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019; 71:793–801.
Article19. Huang DQ, Wilson LA, Behling C, Kleiner DE, Kowdley KV, Dasarathy S, et al. Fibrosis progression rate in biopsy-proven nonalcoholic fatty liver disease among people with diabetes versus people without diabetes: a multicenter study. Gastroenterology. 2023; 165:463–72.
Article20. American Diabetes Association. American Diabetes Association releases a guideline update in NAFLD (non-alcoholic fatty liver disease) and diabetes. Available from: https://www2.diabetes.org/newsroom/press-releases/2023/american-diabetes-association-releases-guideline-update-NAFLD-diabetes (cited 2023 Oct 27).21. Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023; 77:1797–835.
Article22. Sanyal AJ, Castera L, Wong VW. Noninvasive assessment of liver fibrosis in NAFLD. Clin Gastroenterol Hepatol. 2023; 21:2026–39.
Article23. Kanwal F, Shubrook JH, Adams LA, Pfotenhauer K, Wai-Sun Wong V, Wright E, et al. Clinical care pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology. 2021; 161:1657–69.
Article24. Colosimo S, Tan GD, Petroni ML, Marchesini G, Tomlinson JW. Improved glycaemic control in patients with type 2 diabetes has a beneficial impact on NAFLD, independent of change in BMI or glucose lowering agent. Nutr Metab Cardiovasc Dis. 2023; 33:640–8.
Article25. Lee SM, Jung YM, Choi ES, Kwak SH, Koo JN, Oh IH, et al. Metabolic dysfunction-associated fatty liver disease and subsequent development of adverse pregnancy outcomes. Clin Gastroenterol Hepatol. 2022; 20:2542–50.
Article26. Bapat SP, Whitty C, Mowery CT, Liang Y, Yoo A, Jiang Z, et al. Obesity alters pathology and treatment response in inflammatory disease. Nature. 2022; 604:337–42.
Article27. Peiseler M, Schwabe R, Hampe J, Kubes P, Heikenwalder M, Tacke F. Immune mechanisms linking metabolic injury to inflammation and fibrosis in fatty liver disease: novel insights into cellular communication circuits. J Hepatol. 2022; 77:1136–60.28. Pan J, Ding Y, Sun Y, Li Q, Wei T, Gu Y, et al. Associations between adipokines and metabolic dysfunction-associated fatty liver disease using three different diagnostic criteria. J Clin Med. 2023; 12:2126.
Article29. Carter JK, Friedman SL. Hepatic stellate cell-immune interactions in NASH. Front Endocrinol (Lausanne). 2022; 13:867940.
Article30. Xiao Y, Batmanov K, Hu W, Zhu K, Tom AY, Guan D, et al. Hepatocytes demarcated by EphB2 contribute to the progression of nonalcoholic steatohepatitis. Sci Transl Med. 2023; 15:eadc9653.
Article31. Leung H, Long X, Ni Y, Qian L, Nychas E, Siliceo SL, et al. Risk assessment with gut microbiome and metabolite markers in NAFLD development. Sci Transl Med. 2022; 14:eabk0855.
Article32. Kawano Y, Edwards M, Huang Y, Bilate AM, Araujo LP, Tanoue T, et al. Microbiota imbalance induced by dietary sugar disrupts immune-mediated protection from metabolic syndrome. Cell. 2022; 185:3501–19.
Article33. Acharya C, Bajaj JS. Chronic liver diseases and the microbiome-translating our knowledge of gut microbiota to management of chronic liver disease. Gastroenterology. 2021; 160:556–72.
Article34. Meijnikman AS, Davids M, Herrema H, Aydin O, Tremaroli V, Rios-Morales M, et al. Microbiome-derived ethanol in nonalcoholic fatty liver disease. Nat Med. 2022; 28:2100–6.
Article35. Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018; 24:908–22.
Article36. Schwabe RF, Tabas I, Pajvani UB. Mechanisms of fibrosis development in nonalcoholic steatohepatitis. Gastroenterology. 2020; 158:1913–28.
Article37. Rockey DC. Hepatic fibrosis, stellate cells, and portal hypertension. Clin Liver Dis. 2006; 10:459–79.
Article38. Trepo E, Valenti L. Update on NAFLD genetics: from new variants to the clinic. J Hepatol. 2020; 72:1196–209.
Article39. Chen VL, Oliveri A, Miller MJ, Wijarnpreecha K, Du X, Chen Y, et al. PNPLA3 genotype and diabetes identify patients with nonalcoholic fatty liver disease at high risk of incident cirrhosis. Gastroenterology. 2023; 164:966–77.
Article40. Vujkovic M, Ramdas S, Lorenz KM, Guo X, Darlay R, Cordell HJ, et al. A multiancestry genome-wide association study of unexplained chronic ALT elevation as a proxy for nonalcoholic fatty liver disease with histological and radiological validation. Nat Genet. 2022; 54:761–71.
Article41. Rutledge SM, Soper ER, Ma N, Pejaver V, Friedman SL, Branch AD, et al. Association of HSD17B13 and PNPLA3 with liver enzymes and fibrosis in Hispanic/Latino individuals of diverse genetic ancestries. Clin Gastroenterol Hepatol. 2023; 21:2578–87.
Article42. Anstee QM, Darlay R, Cockell S, Meroni M, Govaere O, Tiniakos D, et al. Genome-wide association study of non-alcoholic fatty liver and steatohepatitis in a histologically characterised cohort. J Hepatol. 2020; 73:505–15.
Article43. Tamaki N, Ahlholm N, Luukkonen PK, Porthan K, Sharpton SR, Ajmera V, et al. Risk of advanced fibrosis in first-degree relatives of patients with nonalcoholic fatty liver disease. J Clin Invest. 2022; 132:e162513.
Article44. Huang DQ, Ahlholm N, Luukkonen PK, Porthan K, Amangurbanova M, Madamba E, et al. Development and validation of the nonalcoholic fatty liver disease familial risk score to detect advanced fibrosis: a prospective, multicenter study. Clin Gastroenterol Hepatol. 2024; 22:81–90.45. Kim HS, Xiao X, Byun J, Jun G, DeSantis SM, Chen H, et al. Synergistic associations of PNPLA3 I148M variant, alcohol intake, and obesity with risk of cirrhosis, hepatocellular carcinoma, and mortality. JAMA Netw Open. 2022; 5:e2234221.
Article46. Luukkonen PK, Qadri S, Ahlholm N, Porthan K, Mannisto V, Sammalkorpi H, et al. Distinct contributions of metabolic dysfunction and genetic risk factors in the pathogenesis of non-alcoholic fatty liver disease. J Hepatol. 2022; 76:526–35.
Article47. Tacke F, Puengel T, Loomba R, Friedman SL. An integrated view of anti-inflammatory and antifibrotic targets for the treatment of NASH. J Hepatol. 2023; 79:552–66.
Article48. Harrison SA, Loomba R, Dubourg J, Ratziu V, Noureddin M. Clinical trial landscape in NASH. Clin Gastroenterol Hepatol. 2023; 21:2001–14.
Article49. Dufour JF, Anstee QM, Bugianesi E, Harrison S, Loomba R, Paradis V, et al. Current therapies and new developments in NASH. Gut. 2022; 71:2123–34.
Article50. Bedossa P, Poitou C, Veyrie N, Bouillot JL, Basdevant A, Paradis V, et al. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology. 2012; 56:1751–9.
Article51. Pai RK, Jairath V, Hogan M, Zou G, Adeyi OA, Anstee QM, et al. Reliability of histologic assessment for NAFLD and development of an expanded NAFLD activity score. Hepatology. 2022; 76:1150–63.
Article52. Sanyal AJ, Van Natta ML, Clark J, Neuschwander-Tetri BA, Diehl A, Dasarathy S, et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med. 2021; 385:1559–69.
Article53. Marti-Aguado D, Rodriguez-Ortega A, Mestre-Alagarda C, Bauza M, Valero-Perez E, Alfaro-Cervello C, et al. Digital pathology: accurate technique for quantitative assessment of histological features in metabolic-associated fatty liver disease. Aliment Pharmacol Ther. 2021; 53:160–71.
Article54. Naoumov NV, Brees D, Loeffler J, Chng E, Ren Y, Lopez P, et al. Digital pathology with artificial intelligence analyses provides greater insights into treatment-induced fibrosis regression in NASH. J Hepatol. 2022; 77:1399–409.
Article55. Taylor-Weiner A, Pokkalla H, Han L, Jia C, Huss R, Chung C, et al. A machine learning approach enables quantitative measurement of liver histology and disease monitoring in NASH. Hepatology. 2021; 74:133–47.
Article56. Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017; 14:397–411.
Article57. Troeger JS, Mederacke I, Gwak GY, Dapito DH, Mu X, Hsu CC, et al. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology. 2012; 143:1073–83.
Article58. Cogliati B, Yashaswini CN, Wang S, Sia D, Friedman SL. Friend or foe?: the elusive role of hepatic stellate cells in liver cancer. Nat Rev Gastroenterol Hepatol. 2023; 20:647–61.
Article59. Kisseleva T, Brenner DA. Inactivation of myofibroblasts during regression of liver fibrosis. Cell Cycle. 2013; 12:381–2.
Article60. Amor C, Feucht J, Leibold J, Ho YJ, Zhu C, Alonso-Curbelo D, et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature. 2020; 583:127–32.
Article61. Saviano A, Henderson NC, Baumert TF. Single-cell genomics and spatial transcriptomics: discovery of novel cell states and cellular interactions in liver physiology and disease biology. J Hepatol. 2020; 73:1219–30.62. Filliol A, Saito Y, Nair A, Dapito DH, Yu LX, Ravichandra A, et al. Opposing roles of hepatic stellate cell subpopulations in hepatocarcinogenesis. Nature. 2022; 610:356–65.
Article63. Wang S, Li K, Pickholz E, Dobie R, Matchett KP, Henderson NC, et al. An autocrine signaling circuit in hepatic stellate cells underlies advanced fibrosis in nonalcoholic steatohepatitis. Sci Transl Med. 2023; 15:eadd3949.
Article64. Adler M, Mayo A, Zhou X, Franklin RA, Meizlish ML, Medzhitov R, et al. Principles of cell circuits for tissue repair and fibrosis. iScience. 2020; 23:100841.
Article65. Llovet JM, Willoughby CE, Singal AG, Greten TF, Heikenwalder M, El-Serag HB, et al. Nonalcoholic steatohepatitis-related hepatocellular carcinoma: pathogenesis and treatment. Nat Rev Gastroenterol Hepatol. 2023; 20:487–503.
Article66. Mittal S, El-Serag HB, Sada YH, Kanwal F, Duan Z, Temple S, et al. Hepatocellular carcinoma in the absence of cirrhosis in united states veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016; 14:124–31.
Article67. Kanwal F, Kramer JR, Mapakshi S, Natarajan Y, Chayanupatkul M, Richardson PA, et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology. 2018; 155:1828–37.
Article68. Pfister D, Nunez NG, Pinyol R, Govaere O, Pinter M, Szydlowska M, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021; 592:450–6.69. Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013; 381:468–75.
Article70. Rockey DC, Friedman SL. Fibrosis regression after eradication of hepatitis C virus: from bench to bedside. Gastroenterology. 2021; 160:1502–20.
Article71. Lassailly G, Caiazzo R, Ntandja-Wandji LC, Gnemmi V, Baud G, Verkindt H, et al. Bariatric surgery provides long-term resolution of nonalcoholic steatohepatitis and regression of fibrosis. Gastroenterology. 2020; 159:1290–301.
Article72. Aminian A, Al-Kurd A, Wilson R, Bena J, Fayazzadeh H, Singh T, et al. Association of bariatric surgery with major adverse liver and cardiovascular outcomes in patients with biopsy-proven nonalcoholic steatohepatitis. JAMA. 2021; 326:2031–42.
Article73. Pais R, Aron-Wisnewsky J, Bedossa P, Ponnaiah M, Oppert JM, Siksik JM, et al. Persistence of severe liver fibrosis despite substantial weight loss with bariatric surgery. Hepatology. 2022; 76:456–68.
Article74. Rustgi VK, Li Y, Gupta K, Minacapelli CD, Bhurwal A, Catalano C, et al. Bariatric surgery reduces cancer risk in adults with nonalcoholic fatty liver disease and severe obesity. Gastroenterology. 2021; 161:171–84.
Article75. Fujiwara N, Kubota N, Crouchet E, Koneru B, Marquez CA, Jajoriya AK, et al. Molecular signatures of long-term hepatocellular carcinoma risk in nonalcoholic fatty liver disease. Sci Transl Med. 2022; 14:eabo4474.
Article76. Pellicoro A, Ramachandran P, Iredale JP. Reversibility of liver fibrosis. Fibrogenesis Tissue Repair. 2012; 5(Suppl 1):S26.
Article77. Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A, et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci U S A. 2012; 109:E3186–95.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hepatic Fibrosis and Steatosis in Metabolic Syndrome
- Age serves as the silent architect of FIB-4’s precision in unveiling advanced hepatic fibrosis in MASLD with T2DM: Correspondence to letter to the editor on “Diagnostic accuracy of the fibrosis-4 index for advanced liver fibrosis in nonalcoholic fatty liver disease with type 2 diabetes: a systematic review and meta-analysis”
- Letter: Hepatic Fibrosis and Steatosis in Metabolic Syndrome (J Obes Metab Syndr 2022;31:61-9)
- Pharmacologic Therapy of Hepatic Fibrosis
- Effects of Metabolic Syndrome on Fibrosis in Chronic Viral Hepatitis