J Pathol Transl Med.
2024 Mar;58(2):72-80. 10.4132/jptm.2024.01.23.
TRPS1 expression in non-melanocytic cutaneous neoplasms: an immunohistochemical analysis of 200 cases
- Affiliations
-
- 1Department of Pathology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
- 2Department of Biostatistics, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
- KMID: 2553474
- DOI: http://doi.org/10.4132/jptm.2024.01.23
Abstract
- Background
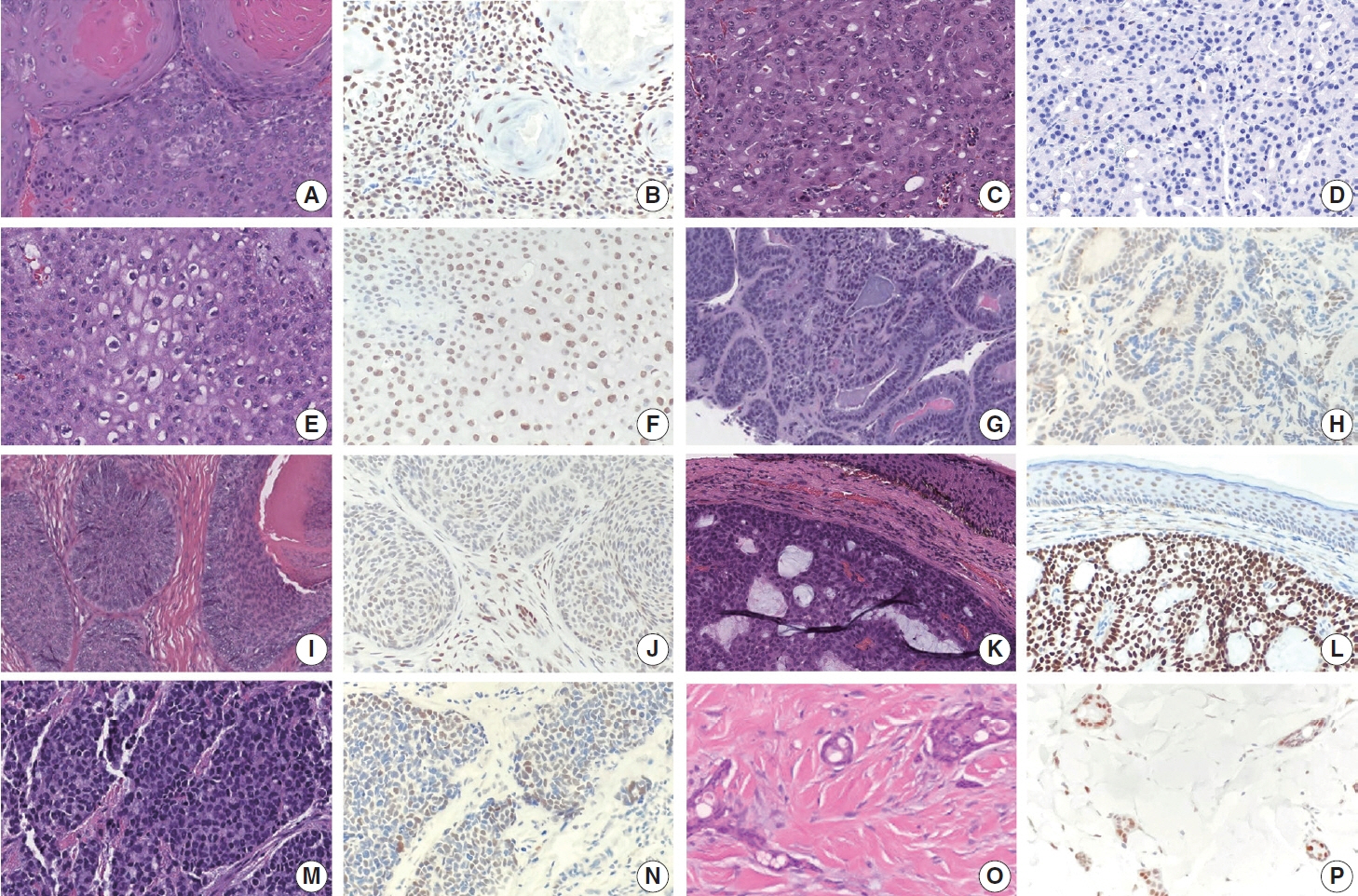
Although trichorhinophalangeal syndrome type 1 (TRPS1) was initially thought to be highly sensitive and specific for carcinomas and mesenchymal tumors of mammary origin, more recent data suggest its expression is not limited to breast neoplasms but also can be seen in other cutaneous neoplasms, such as extramammary Paget disease and squamous cell carcinoma (SCC) in situ.
Methods
Two-hundred cases of non-melanocytic cutaneous neoplasm, including basal cell carcinomas (BCCs) (n = 41), SCCs (n = 35), Merkel cell carcinomas (MCCs) (n = 25), and adnexal neoplasms (n = 99), were tested for TRPS1 expression using a monoclonal anti- TRPS1 rabbit anti-human antibody.
Results
TRPS1 expression was present in almost all cases of SCC (94%), with a median H-score of 200, while it was either absent or only focally present in most BCCs (90%), with a median H-score of 5. The difference between BCCs and SCCs in H-score was significant (p < .001). All MCCs (100%) lacked TRPS1 expression. TRPS1 expression was frequently seen in most adnexal neoplasms, benign and malignant, in variable intensity and proportion but was consistently absent in apocrine carcinomas. All endocrine mucin-producing sweat gland carcinomas (EMPSGCs) (100%, 6/6) showed diffuse and strong TRPS1 immunoreactivity, with a median H-score of 300, which was significantly different (p < .001) than that of BCCs.
Conclusions
Our study shows that TRPS1 may be an effective discriminatory marker for BCCs and SCCs. It also has a role in distinguishing BCCs from EMPSGCs.
Keyword
Figure
Reference
-
References
1. Momeni P, Glockner G, Schmidt O, et al. Mutations in a new gene, encoding a zinc-finger protein, cause tricho-rhino-phalangeal syndrome type I. Nat Genet. 2000; 24:71–4.2. Malik TH, Von Stechow D, Bronson RT, Shivdasani RA. Deletion of the GATA domain of TRPS1 causes an absence of facial hair and provides new insights into the bone disorder in inherited trichorhino-phalangeal syndromes. Mol Cell Biol. 2002; 22:8592–600.3. Ludecke HJ, Schaper J, Meinecke P, et al. Genotypic and phenotypic spectrum in tricho-rhino-phalangeal syndrome types I and III. Am J Hum Genet. 2001; 68:81–91.4. Ai D, Yao J, Yang F, et al. TRPS1: a highly sensitive and specific marker for breast carcinoma, especially for triple-negative breast cancer. Mod Pathol. 2021; 34:710–9.5. Wang J, Wang WL, Sun H, et al. Expression of TRPS1 in phyllodes tumor and sarcoma of the breast. Hum Pathol. 2022; 121:73–80.6. Cho WC, Ding Q, Wang WL, et al. Immunohistochemical expression of TRPS1 in mammary Paget disease, extramammary Paget disease, and their close histopathologic mimics. J Cutan Pathol. 2023; 50:434–40.7. Liu YA, Collins K, Aung PP, et al. TRPS1 expression in primary and secondary extramammary Paget diseases: an immunohistochemical analysis of 93 cases. Hum Pathol. 2024; 143:5–9.8. Cho WC, Nagarajan P, Ding Q, Prieto VG, Torres-Cabala CA. Trichorhinophalangeal syndrome type 1-positive cells in breast dermal granulation tissues and scars: a potential diagnostic pitfall. Am J Dermatopathol. 2022; 44:964–7.9. Cloutier JM, Ingram DR, Wani K, Lazar AJ, Wang WL. Frequent TRPS1 expression in synovial sarcoma is associated with SS18-SSX fusion oncoprotein activity. Hum Pathol. 2022; 130:88–94.10. Youssef KK, Van Keymeulen A, Lapouge G, et al. Identification of the cell lineage at the origin of basal cell carcinoma. Nat Cell Biol. 2010; 12:299–305.11. George E, Swanson PE, Wick MR. Neuroendocrine differentiation in basal cell carcinoma: an immunohistochemical study. Am J Dermatopathol. 1989; 11:131–5.12. Liu YA, Cho WC. TRPS1 expression in endocrine mucin-producing sweat gland carcinoma: diagnostic utility and pitfalls. Am J Dermatopathol. 2024; 46:133–5.13. Ivan D, Nash JW, Prieto VG, et al. Use of p63 expression in distinguishing primary and metastatic cutaneous adnexal neoplasms from metastatic adenocarcinoma to skin. J Cutan Pathol. 2007; 34:474–80.14. Plaza JA, Ortega PF, Stockman DL, Suster S. Value of p63 and podoplanin (D2-40) immunoreactivity in the distinction between primary cutaneous tumors and adenocarcinomas metastatic to the skin: a clinicopathologic and immunohistochemical study of 79 cases. J Cutan Pathol. 2010; 37:403–10.15. Mahalingam M, Nguyen LP, Richards JE, Muzikansky A, Hoang MP. The diagnostic utility of immunohistochemistry in distinguishing primary skin adnexal carcinomas from metastatic adenocarcinoma to skin: an immunohistochemical reappraisal using cytokeratin 15, nestin, p63, D2-40, and calretinin. Mod Pathol. 2010; 23:713–9.16. Zengin HB, Bui CM, Rybski K, Pukhalskaya T, Yildiz B, Smoller BR. TRPS1 is differentially expressed in a variety of malignant and benign cutaneous sweat gland neoplasms. Dermatopathology (Basel). 2023; 10:75–85.17. Beer TW, Shepherd P, Theaker JM. Ber EP4 and epithelial membrane antigen aid distinction of basal cell, squamous cell and basosquamous carcinomas of the skin. Histopathology. 2000; 37:218–23.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunohistochemical Study on the Expression of Mutated p53 Protein and Bcl-2 Protein in Melanocytic Lesions of Skin
- Alteration of Apoptosis-Related Proteins (Apaf-1, Caspase-9, Bcl-2, p53, and Survivin) According to Malignant Progression in Cutaneous Melanocytic Lesions
- Three Cases of Malignant Melanoma Possibly Arising in a Long Standing Melanocytic Nevus
- Immunohistochemical Expression of MMP-2 and MMP-9 Metalloproteinases in Melanocytic Nevi and Malignant Melanomas
- Immunohistochemical Expression of p16 and p21 in Melanocytic Nevi and Malignant Melanoma