Obstet Gynecol Sci.
2024 Mar;67(2):169-185. 10.5468/ogs.23274.
Vulval premalignant lesions: a review article
- Affiliations
-
- 1Department of Obstetrics and Gynaecology, Vardhman Mahavir Medical College and Safdarjung Hospital, New Delhi, India
- 2Department of Pathology, Vardhman Mahavir Medical College and Safdarjung Hospital, New Delhi, India
- 3Department of General Surgery, Vardhman Mahavir Medical College and Safdarjung Hospital, New Delhi, India
- KMID: 2553425
- DOI: http://doi.org/10.5468/ogs.23274
Abstract
- Vulvar intraepithelial neoplasia (VIN) is a noninvasive squamous lesion that is a precursor of vulvar squamous cell cancer. Currently, no screening tests are available for detecting VIN, and a biopsy is performed to confirm the clinical diagnosis. Despite sharing many risk factors with cervical intraepithelial neoplasia, the diagnosis of VIN is poses challenges, contributing to its increasing prevalence. This study aimed to analyze the underlying risk factors that contribute to the development of VIN, identify specific populations at risk, and define appropriate treatment approaches. Differentiated VIN (dVIN) and usual VIN (uVIN) are the classifications of VIN. While dVIN is associated with other vulvar inflammatory disorders, such as lichen sclerosis, the more prevalent uVIN is associated with an underlying human papillomavirus infection. Patients with differentiated VIN have an increased risk of developing invasive malignancies. Few effective surveillance or management techniques exist for vulvar intraepithelial neoplasia, a preinvasive neoplasm of the vulva. For suspicious lesions, a thorough examination and focused biopsy are necessary. Depending on the specific needs of each patient, a combination of surgical and medical approaches can be used.
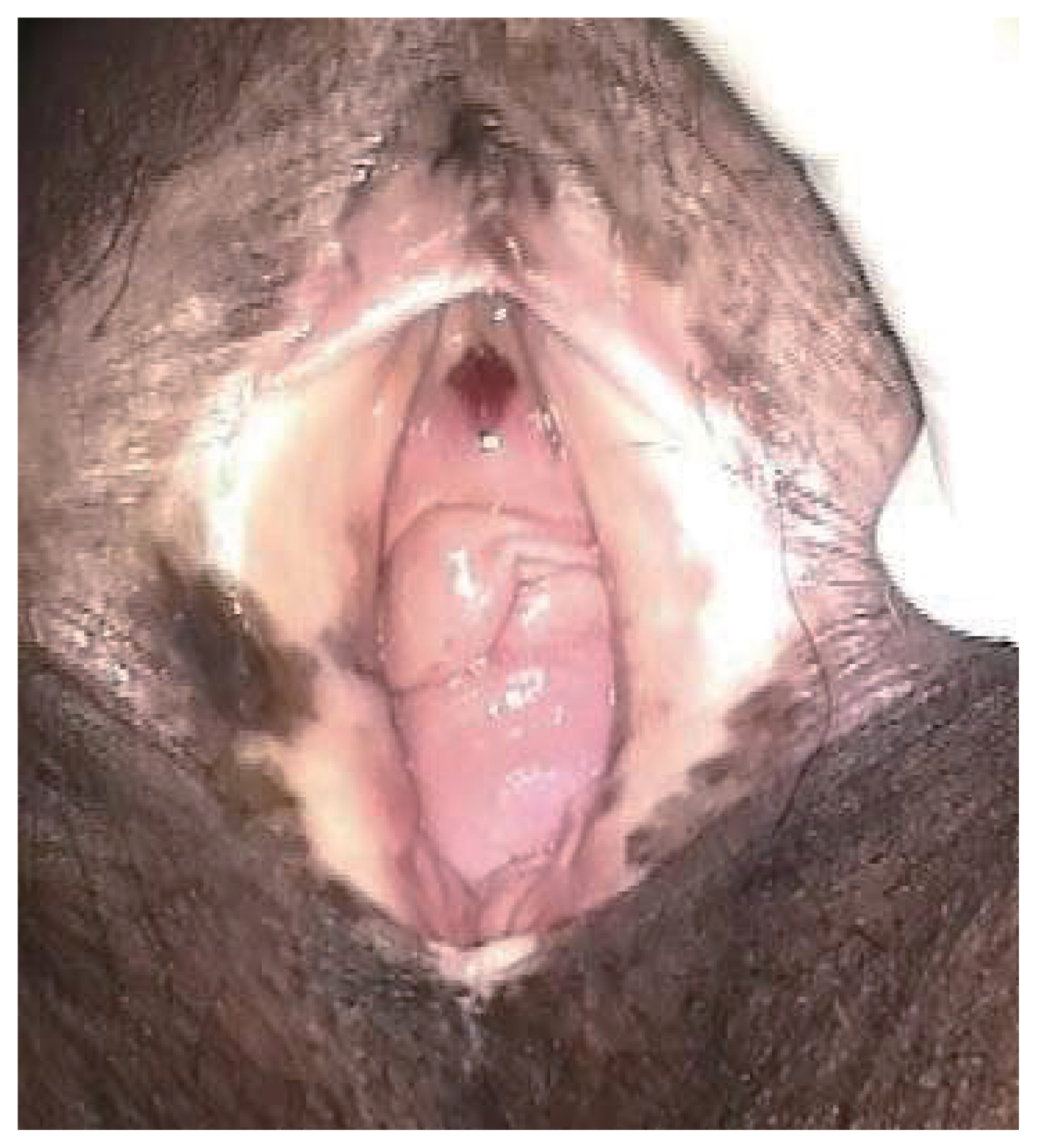
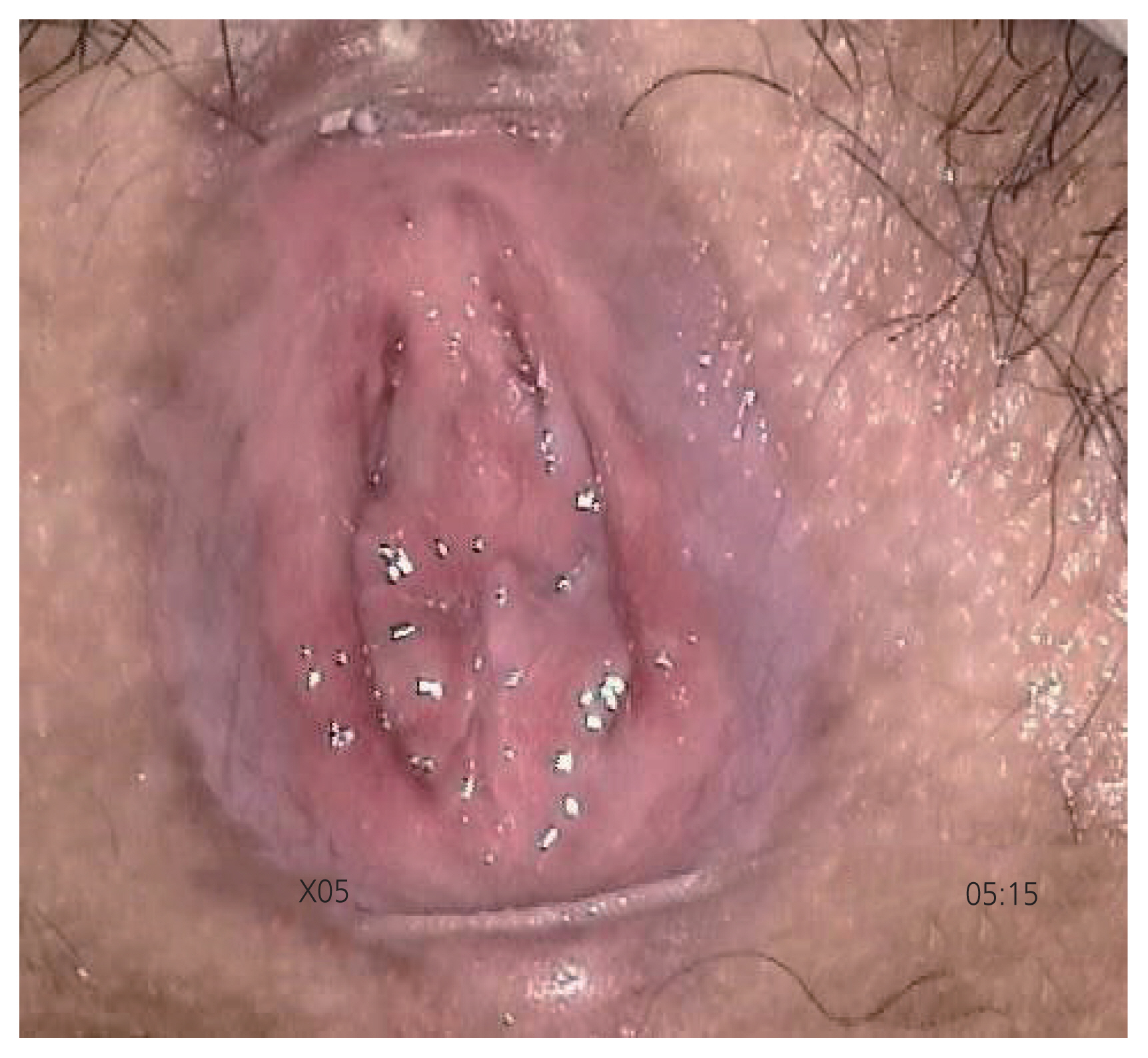
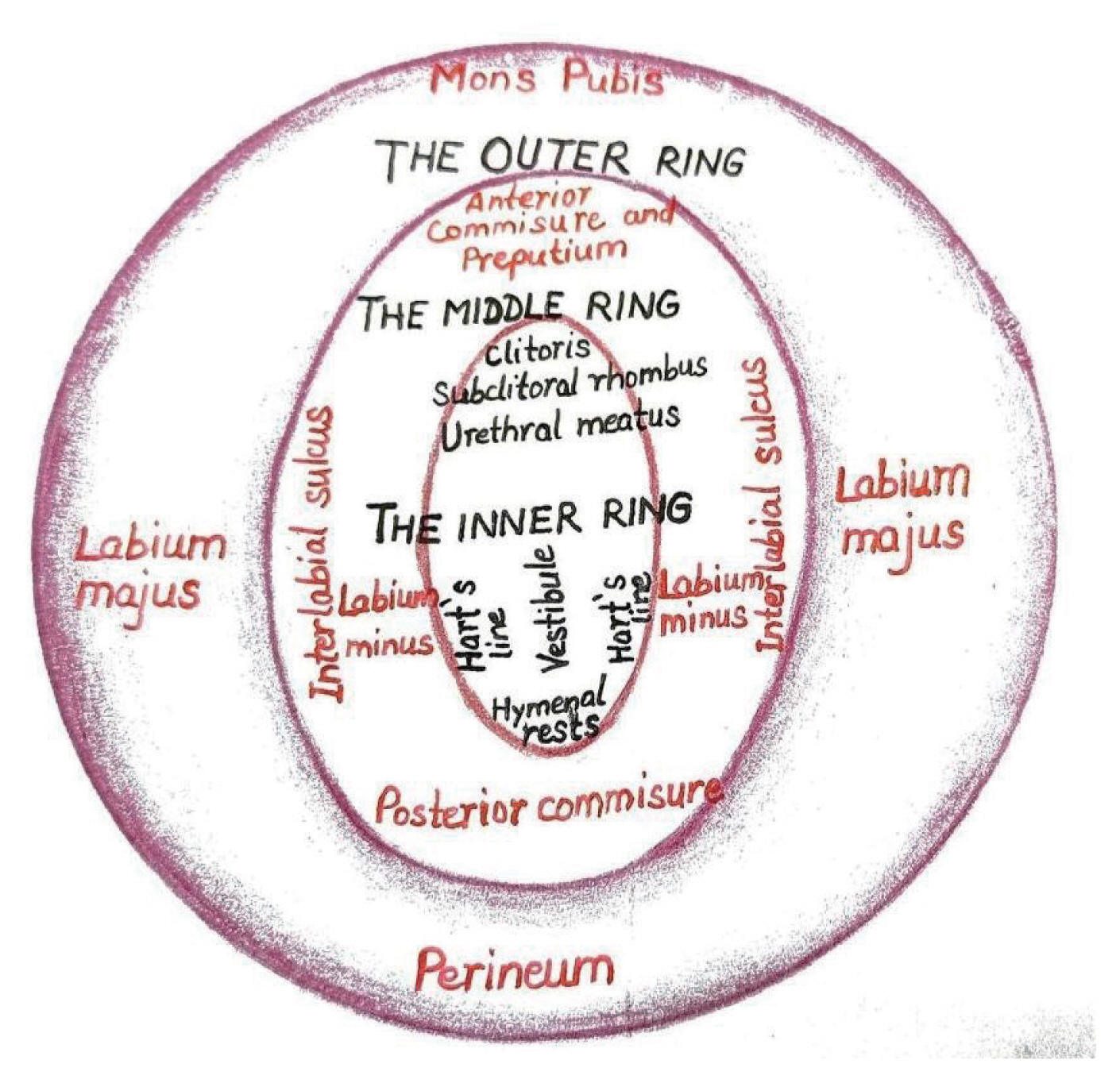
Figure
Reference
-
References
1. Preti M, Van Seters M, Sideri M, Van Beurden M. Squamous vulvar intraepithelial neoplasia. Clin Obstet Gynecol. 2005; 48:845–61.2. New nomenclature for vulvar disease. Obstet Gynecol. 1976; 47:122–4.3. Wilkinson EJ, Kneale B, Lynch PJ. Report of the ISSVD terminology committee. J Reprod Med. 1986; 31:973–4.4. Sideri M, Jones RW, Wilkinson EJ, Preti M, Heller DS, Scurry J, et al. Squamous vulvar intraepithelial neoplasia: 2004 modified terminology, ISSVD Vulvar Oncology Subcommittee. J Reprod Med. 2005; 50:807–10.5. Scurry J, Wilkinson EJ. Review of terminology of precursors of vulvar squamous cell carcinoma. J Low Genit Tract Dis. 2006; 10:161–9.6. Wilkinson EJ, Teixeira MR. Tumors of the vulva. Tavassoli FA, Devilee P, editors. Pathology and genetics of tumours of the breast and female genital organs World Health Organization classification of tumours. 4th ed. Lyon: IARC Press;2003. p. 313–34.7. Giuliano AR, Tortolero-Luna G, Ferrer E, Burchell AN, de Sanjose S, Kjaer SK, et al. Epidemiology of human papillomavirus infection in men, cancers other than cervical and benign conditions. Vaccine. 2008; 26(Suppl 10):K17–28.8. van de Nieuwenhof HP, Massuger LF, van der Avoort IA, Bekkers RL, Casparie M, Abma W, et al. Vulvar squamous cell carcinoma development after diagnosis of VIN increases with age. Eur J Cancer. 2009; 45:851–6.9. Hart WR. Vulvar intraepithelial neoplasia: historical aspects and current status. Int J Gynecol Pathol. 2001; 20:16–30.10. Scurry J, Campion M, Scurry B, Kim SN, Hacker N. Pathologic audit of 164 consecutive cases of vulvar intraepithelial neoplasia. Int J Gynecol Pathol. 2006; 25:176–81.11. Yang B, Hart WR. Vulvar intraepithelial neoplasia of the simplex (differentiated) type: a clinicopathologic study including analysis of HPV and p53 expression. Am J Surg Pathol. 2000; 24:429–41.12. Darragh TM, Colgan TJ, Cox JT, Heller DS, Henry MR, Luff RD, et al. The lower anogenital squamous terminology standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. J Low Genit Tract Dis. 2012; 16:205–42.13. World Health Organization. International classification of diseases for mortality and morbidity statistics (11th revision) [Internet]. Geneva: World Health Organization;c2018. [cited 2018 Jun 18]. Available from: https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/913387730.14. Bornstein J, Bogliatto F, Haefner HK, Stockdale CK, Preti M, Bohl TG, et al. The 2015 International Society for the Study of Vulvovaginal Disease (ISSVD) terminology of vulvar squamous intraepithelial lesions. J Low Genit Tract Dis. 2016; 20:11–4.15. Crum C, Herrington C, McCluggage W. Tumours of the vulva; epithelial tumors. Kurman RJ, Carcangiu ML, Herrington CS, editors. WHO classification of tumours of female reproductive organs. 4th ed. Lyon: IARC Press;2014. p. 229–52.16. WHO Classification of Tumours Editorial Board. Female genital tumours [Internet]. Lyon: WHO Classification of Tumours Editorial Board;c2020. [cited 2020 Sep 30]. Available from: https://tumourclassification.iarc.who.int/chapters/34.17. Lebreton M, Carton I, Brousse S, Lavoué V, Body G, Levêque J, et al. Vulvar intraepithelial neoplasia: classification, epidemiology, diagnosis, and management. J Gynecol Obstet Hum Reprod. 2020; 49:101801.18. Lukasiewicz E, Aractingi S, Flahault A. Incidence and management of condylomata acuminata by French general physicians. Ann Dermatol Venereol. 2002; 129:991–6.19. Monsonégo J, Breugelmans JG, Bouée S, Lafuma A, Bénard S, Rémy V. Anogenital warts incidence, medical management and costs in women consulting gynaecologists in France. Gynecol Obstet Fertil. 2007; 35:107–13.20. van Seters M, van Beurden M, de Craen AJ. Is the assumed natural history of vulvar intraepithelial neoplasia III based on enough evidence? A systematic review of 3322 published patients. Gynecol Oncol. 2005; 97:645–51.21. Jones RW, Rowan DM, Stewart AW. Vulvar intraepithelial neoplasia: aspects of the natural history and outcome in 405 women. Obstet Gynecol. 2005; 106:1319–26.22. Thuijs NB, van Beurden M, Bruggink AH, Steenbergen RDM, Berkhof J, Bleeker MCG. Vulvar intraepithelial neoplasia: incidence and long-term risk of vulvar squamous cell carcinoma. Int J Cancer. 2021; 148:90–8.23. de Sanjosé S, Alemany L, Ordi J, Tous S, Alejo M, Bigby SM, et al. Worldwide human papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. Eur J Cancer. 2013; 49:3450–61.24. Clifford GM, Georges D, Shiels MS, Engels EA, Albuquerque A, Poynten IM, et al. A meta-analysis of anal cancer incidence by risk group: toward a unified anal cancer risk scale. Int J Cancer. 2021; 148:38–47.25. Saleem AM, Paulus JK, Shapter AP, Baxter NN, Roberts PL, Ricciardi R. Risk of anal cancer in a cohort with human papillomavirus-related gynecologic neoplasm. Obstet Gynecol. 2011; 117:643–9.26. Faber MT, Sand FL, Albieri V, Norrild B, Kjaer SK, Verdoodt F. Prevalence and type distribution of human papillomavirus in squamous cell carcinoma and intraepithelial neoplasia of the vulva. Int J Cancer. 2017; 141:1161–9.27. Rakislova N, Saco A, Sierra A, Del Pino M, Ordi J. Role of human papillomavirus in vulvar cancer. Adv Anat Pathol. 2017; 24:201–14.28. Thuijs NB, Berkhof J, Özer M, Duin S, van Splunter AP, Snoek BC, et al. DNA methylation markers for cancer risk prediction of vulvar intraepithelial neoplasia. Int J Cancer. 2021; 148:2481–8.29. Swarts DRA, Voorham QJM, van Splunter AP, Wilting SM, Sie D, Pronk D, et al. Molecular heterogeneity in human papillomavirus-dependent and -independent vulvar carcinogenesis. Cancer Med. 2018; 7:4542–53.30. Bleeker MC, Visser PJ, Overbeek LI, van Beurden M, Berkhof J. Lichen sclerosus: incidence and risk of vulvar squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2016; 25:1224–30.31. Del Pino M, Rodriguez-Carunchio L, Ordi J. Pathways of vulvar intraepithelial neoplasia and squamous cell carcinoma. Histopathology. 2013; 62:161–75.32. Nooij LS, Ter Haar NT, Ruano D, Rakislova N, van Wezel T, Smit VTHBM, et al. Genomic characterization of vulvar (pre)cancers identifies distinct molecular subtypes with prognostic significance. Clin Cancer Res. 2017; 23:6781–9.33. Watkins JC, Howitt BE, Horowitz NS, Ritterhouse LL, Dong F, MacConaill LE, et al. Differentiated exophytic vulvar intraepithelial lesions are genetically distinct from keratinizing squamous cell carcinomas and contain mutations in PIK3CA. Mod Pathol. 2017; 30:448–58.34. Tessier-Cloutier B, Kortekaas KE, Thompson E, Pors J, Chen J, Ho J, et al. Major p53 immunohistochemical patterns in in situ and invasive squamous cell carcinomas of the vulva and correlation with TP53 mutation status. Mod Pathol. 2020; 33:1595–605.35. Fatalska A, Rusetska N, Bakuła-Zalewska E, Kowalik A, Zięba S, Wroblewska A, et al. Inflammatory proteins HMGA2 and PRTN3 as drivers of vulvar squamous cell carcinoma progression. Cancers (Basel). 2020; 13:27.36. Hoang LN, Park KJ, Soslow RA, Murali R. Squamous precursor lesions of the vulva: current classification and diagnostic challenges. Pathology. 2016; 48:291–302.37. Regauer S, Reich O, Eberz B. Vulvar cancers in women with vulvar lichen planus: a clinicopathological study. J Am Acad Dermatol. 2014; 71:698–707.38. McNally OM, Mulvany NJ, Pagano R, Quinn MA, Rome RM. VIN 3: a clinicopathologic review. Int J Gynecol Cancer. 2002; 12:490–5.39. van de Nieuwenhof HP, van der Avoort IA, de Hullu JA. Review of squamous premalignant vulvar lesions. Crit Rev Oncol Hematol. 2008; 68:131–56.40. Khan AM, Freeman-Wang T, Pisal N, Singer A. Smoking and multicentric vulval intraepithelial neoplasia. J Obstet Gynaecol. 2009; 29:123–5.41. van Beurden M, van der Vange N, de Craen AJ, Tjong-AHung SP, ten Kate FJ, ter Schegget J, et al. Normal findings in vulvar examination and vulvoscopy. Br J Obstet Gynaecol. 1997; 104:320–4.42. Albuquerque A, Rios E, Schmitt F. Recommendations favoring anal cytology as a method for anal cancer screening: a systematic review. Cancers (Basel). 2019; 11:1942.43. Preti M, Scurry J, Marchitelli CE, Micheletti L. Vulvar intraepithelial neoplasia. Best Pract Res Clin Obstet Gynaecol. 2014; 28:1051–62.44. Jin C, Liang S. Differentiated vulvar intraepithelial neoplasia: a brief review of clinicopathologic features. Arch Pathol Lab Med. 2019; 143:768–71.45. Kesic V. Colposcopy of the vulva, perineum and anal canal. Bösze P, Luesley D, editors. EAGC course book of colposcopy. 14th ed. Waltham: Nova Biomedical;2004. p. 126–63.46. Neill S, Lewis FM. Ridley’s the vulva. 3rd ed. London: John Wiley & Sons;2009.47. Heller DS, Day T, Allbritton JI, Scurry J, Radici G, Welch K, et al. Diagnostic criteria for differentiated vulvar intraepithelial neoplasia and vulvar aberrant maturation. J Low Genit Tract Dis. 2021; 25:57–70.48. Day T, Wilkinson E, Rowan D, Scurry J. Clinicopathologic diagnostic criteria for vulvar lichen planus. J Low Genit Tract Dis. 2020; 24:317–29.49. Terlou A, van Seters M, Kleinjan A, Heijmans-Antonissen C, Santegoets LA, Beckmann I, et al. Imiquimod-induced clearance of HPV is associated with normalization of immune cell counts in usual type vulvar intraepithelial neoplasia. Int J Cancer. 2010; 127:2831–40.50. van Seters M, Beckmann I, Heijmans-Antonissen C, van Beurden M, Ewing PC, Zijlstra FJ, et al. Disturbed patterns of immunocompetent cells in usual-type vulvar intraepithelial neoplasia. Cancer Res. 2008; 68:6617–22.51. van Esch EM, van Poelgeest MI, Trimbos JB, Fleuren GJ, Jordanova ES, van der Burg SH. Intraepithelial macrophage infiltration is related to a high number of regulatory T cells and promotes a progressive course of HPV-induced vulvar neoplasia. Int J Cancer. 2015; 136:E85–94.52. Uyttenhove C, Pilotte L, Théate I, Stroobant V, Colau D, Parmentier N, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003; 9:1269–74.53. Abdulrahman Z, Kortekaas KE, De Vos Van Steenwijk PJ, Van Der Burg SH, Van Poelgeest MI. The immune microenvironment in vulvar (pre)cancer: review of literature and implications for immunotherapy. Expert Opin Biol Ther. 2018; 18:1223–33.54. Lawrie TA, Nordin A, Chakrabarti M, Bryant A, Kaushik S, Pepas L. Medical and surgical interventions for the treatment of usual-type vulval intraepithelial neoplasia. Cochrane Database Syst Rev. 2016; 2016:CD01. 1837.55. Chin S, Scurry J, Bradford J, Lee G, Fischer G. Association of topical corticosteroids with reduced vulvar squamous cell carcinoma recurrence in patients with vulvar lichen sclerosus. JAMA Dermatol. 2020; 156:813–4.56. Te Grootenhuis NC, Pouwer AW, de Bock GH, Hollema H, Bulten J, van der Zee AGJ, et al. Prognostic factors for local recurrence of squamous cell carcinoma of the vulva: a systematic review. Gynecol Oncol. 2018; 148:622–31.57. Yap JK, Fox R, Leonard S, Ganesan R, Kehoe ST, Dawson CW, et al. Adjacent lichen sclerosis predicts local recurrence and second field tumour in women with vulvar squamous cell carcinoma. Gynecol Oncol. 2016; 142:420–6.58. Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015; 151:1061–7.59. Bigby SM, Eva LJ, Fong KL, Jones RW. The natural history of vulvar intraepithelial neoplasia, differentiated type: evidence for progression and diagnostic challenges. Int J Gynecol Pathol. 2016; 35:574–84.60. Regauer S. Residual anogenital lichen sclerosus after cancer surgery has a high risk for recurrence: a clinicopathological study of 75 women. Gynecol Oncol. 2011; 123:289–94.61. Athavale R, Naik R, Godfrey KA, Cross P, Hatem MH, de Barros Lopes A. Vulvar intraepithelial neoplasia--the need for auditable measures of management. Eur J Obstet Gynecol Reprod Biol. 2008; 137:97–102.62. Bradbury M, Cabrera S, García-Jiménez A, Franco-Camps S, Sánchez-Iglesias JL, Díaz-Feijoo B, et al. Vulvar intraepithelial neoplasia: clinical presentation, management and outcomes in women infected with HIV. AIDS. 2016; 30:859–68.63. Bruchim I, Gotlieb WH, Mahmud S, Tunitsky E, Grzywacz K, Ferenczy A. HPV-related vulvar intraepithelial neoplasia: outcome of different management modalities. Int J Gynaecol Obstet. 2007; 99:23–7.64. Fehr MK, Baumann M, Mueller M, Fink D, Heinzl S, Imesch P, et al. Disease progression and recurrence in women treated for vulvovaginal intraepithelial neoplasia. J Gynecol Oncol. 2013; 24:236–41.65. Frega A, Sopracordevole F, Scirpa P, Biamonti A, Lorenzon L, Scarani S, et al. The re-infection rate of high-risk HPV and the recurrence rate of vulvar intraepithelial neoplasia (VIN) usual type after surgical treatment. Med Sci Monit. 2011; 17:CR532–5.66. Leufflen L, Baermann P Jr, Rauch P, Routiot T, Bezdetnava L, Guillemin F, et al. Treatment of vulvar intraepithelial neoplasia with CO(2) laser vaporization and excision surgery. J Low Genit Tract Dis. 2013; 17:446–51.67. van Esch EM, Dam MC, Osse ME, Putter H, Trimbos BJ, Fleuren G, et al. Clinical characteristics associated with development of recurrence and progression in usual-type vulvar intraepithelial neoplasia. Int J Gynecol Cancer. 2013; 23:1476–83.68. Wallbillich JJ, Rhodes HE, Milbourne AM, Munsell MF, Frumovitz M, Brown J, et al. Vulvar intraepithelial neoplasia (VIN 2/3): comparing clinical outcomes and evaluating risk factors for recurrence. Gynecol Oncol. 2012; 127:312–5.69. Miller BE. Vulvar intraepithelial neoplasia treated with cavitational ultrasonic surgical aspiration. Gynecol Oncol. 2002; 85:114–8.70. De Witte CJ, van de Sande AJ, van Beekhuizen HJ, Koeneman MM, Kruse AJ, Gerestein CG. Imiquimod in cervical, vaginal and vulvar intraepithelial neoplasia: a review. Gynecol Oncol. 2015; 139:377–84.71. van Seters M, Fons G, van Beurden M. Imiquimod in the treatment of multifocal vulvar intraepithelial neoplasia 2/3. Results of a pilot study. J Reprod Med. 2002; 47:701–5.72. Mathiesen O, Buus SK, Cramers M. Topical imiquimod can reverse vulvar intraepithelial neoplasia: a randomised, double-blinded study. Gynecol Oncol. 2007; 107:219–22.73. van Seters M, van Beurden M, ten Kate FJ, Beckmann I, Ewing PC, Eijkemans MJ, et al. Treatment of vulvar intraepithelial neoplasia with topical imiquimod. N Engl J Med. 2008; 358:1465–73.74. Tristram A, Hurt CN, Madden T, Powell N, Man S, Hibbitts S, et al. Activity, safety, and feasibility of cidofovir and imiquimod for treatment of vulval intraepithelial neoplasia (RT3VIN): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2014; 15:1361–8.75. Hurt CN, Jones S, Madden TA, Fiander A, Nordin AJ, Naik R, et al. Recurrence of vulval intraepithelial neoplasia following treatment with cidofovir or imiquimod: results from a multicentre, randomised, phase II trial (RT-3VIN). BJOG. 2018; 125:1171–7.76. Jones SEF, Hibbitts S, Hurt CN, Bryant D, Fiander AN, Powell N, et al. Human papillomavirus DNA methylation predicts response to treatment using cidofovir and imiquimod in vulval intraepithelial neoplasia 3. Clin Cancer Res. 2017; 23:5460–8.77. Westermann C, Fischer A, Clad A. Treatment of vulvar intraepithelial neoplasia with topical 5% imiquimod cream. Int J Gynaecol Obstet. 2013; 120:266–70.78. Le T, Menard C, Hicks-Boucher W, Hopkins L, Weberpals J, Fung-Kee-Fung M. Final results of a phase 2 study using continuous 5% imiquimod cream application in the primary treatment of high-grade vulva intraepithelial neoplasia. Gynecol Oncol. 2007; 106:579–84.79. Fehr MK, Hornung R, Degen A, Schwarz VA, Fink D, Haller U, et al. Photodynamic therapy of vulvar and vaginal condyloma and intraepithelial neoplasia using topically applied 5-aminolevulinic acid. Lasers Surg Med. 2002; 30:273–9.80. Zawislak A, Donnelly RF, McCluggage WG, Price JH, McClelland HR, Woolfson AD, et al. Clinical and immunohistochemical assessment of vulval intraepithelial neoplasia following photodynamic therapy using a novel bioadhesive patch-type system loaded with 5-aminolevulinic acid. Photodiagnosis Photodyn Ther. 2009; 6:28–40.81. Buchanan TR, Zamorano AS, Massad LS, Liu J, Thaker PH, Powell MA, et al. Risk of cervical and vaginal dysplasia after surgery for vulvar intraepithelial neoplasia or cancer: a 6 year follow-up study. Gynecol Oncol. 2019; 155:88–92.82. Ait Menguellet S, Collinet P, Houfflin Debarge V, Nayama M, Vinatier D, Leroy JL. Management of multicentric lesions of the lower genital tract. Eur J Obstet Gynecol Reprod Biol. 2007; 132:116–20.83. Garland SM, Joura EA, Ault KA, Bosch FX, Brown DR, Castellsagué X, et al. Human papillomavirus genotypes from vaginal and vulvar intraepithelial neoplasia in females 15–26 years of age. Obstet Gynecol. 2018; 132:261–70.84. Giuliano AR, Joura EA, Garland SM, Huh WK, Iversen OE, Kjaer SK, et al. Nine-valent HPV vaccine efficacy against related diseases and definitive therapy: comparison with historic placebo population. Gynecol Oncol. 2019; 154:110–7.85. Joura EA, Leodolter S, Hernandez-Avila M, Wheeler CM, Perez G, Koutsky LA, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007; 369:1693–702.86. Micheletti L, Preti M, Radici G, Boveri S, Di Pumpo O, Privitera SS, et al. Vulvar lichen sclerosus and neoplastic transformation: a retrospective study of 976 cases. J Low Genit Tract Dis. 2016; 20:180–3.87. Corazza M, Borghi A, Gafà R, Ghirardi C, Ferretti S. Risk of vulvar carcinoma in women affected with lichen sclerosus: results of a cohort study. J Dtsch Dermatol Ges. 2019; 17:1069–71.88. Brickman C, Palefsky JM. Human papillomavirus in the HIV-infected host: epidemiology and pathogenesis in the antiretroviral era. Curr HIV/AIDS Rep. 2015; 12:6–15.89. Konopnicki D, De Wit S, Clumeck N. HPV and HIV coinfection: a complex interaction resulting in epidemiological, clinical and therapeutic implications. Future Virol. 2013; 8:903–15.90. Reinholdt K, Thomsen LT, Dehlendorff C, Larsen HK, Sørensen SS, Haedersdal M, et al. Human papillomavirus-related anogenital premalignancies and cancer in renal transplant recipients: a Danish nationwide, registry-based cohort study. Int J Cancer. 2020; 146:2413–22.91. Preti M, Rotondo JC, Holzinger D, Micheletti L, Gallio N, McKay-Chopin S, et al. Role of human papillomavirus infection in the etiology of vulvar cancer in Italian women. Infect Agent Cancer. 2020; 15:20.92. Meeuwis KA, Melchers WJ, Bouten H, van de Kerkhof PC, Hinten F, Quint WG, et al. Anogenital malignancies in women after renal transplantation over 40 years in a single center. Transplantation. 2012; 93:914–22.93. Chin-Hong PV. Human papillomavirus in kidney transplant recipients. Semin Nephrol. 2016; 36:397–404.94. Chin-Hong PV, Reid GE. Human papillomavirus infection in solid organ transplant recipients: guidelines from the American Society of Transplantation infectious diseases community of practice. Clin Transplant. 2019; 33:e13590.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Crohn's Disease of the Vulva Occurring in Siblings
- A Case of Prepubertal Vulval Fibroma
- A Statistical Study of Cutaneous Malignant Tumors and Premalignant Lesions in Southeastern Gyeonggi-do Province over an 11-year Period (2006~2016)
- A Clinical Observation of Cutaneous Malignant Tumors and Premalignant Lesions in Gangwon Province over 10 Years (1999~2008)
- Management of skin lesion of female external genitalia