Ann Lab Med.
2024 Mar;44(2):126-134. 10.3343/alm.2023.0214.
The Use of Bone-Turnover Markers in Asia-Pacific Populations
- Affiliations
-
- 1Department of Clinical Biochemistry, Fiona Stanley Hospital, Perth, Australia
- 2Department of Pathology, Faculty of Medicine & Health Sciences, Universiti Putra Malaysia, Serdang, Selangor, Malaysia
- 3Engineering Cluster, Singapore Institute of Technology, Singapore
- 4Department of Laboratory Medicine, National University Hospital, Singapore
- KMID: 2553378
- DOI: http://doi.org/10.3343/alm.2023.0214
Abstract
- Bone-turnover marker (BTM) measurements in the blood or urine reflect the bone-remodeling rate and may be useful for studying and clinically managing metabolic bone diseases. Substantial evidence supporting the diagnostic use of BTMs has accumulated in recent years, together with the publication of several guidelines. Most clinical trials and observational and reference-interval studies have been performed in the Northern Hemisphere and have mainly involved Caucasian populations. This review focuses on the available data for populations from the Asia-Pacific region and offers guidance for using BTMs as diagnostic biomarkers in these populations. The procollagen I N-terminal propeptide and β-isomerized C-terminal telopeptide of type-I collagen (measured in plasma) are reference BTMs used for investigating osteoporosis in clinical settings. Premenopausal reference intervals (established for use with Asia-Pacific populations) and reference change values and treatment targets (used to monitor osteoporosis treatment) help guide the management of osteoporosis. Measuring BTMs that are not affected by renal failure, such as the bone-specific isoenzyme alkaline phosphatase and tartrate-resistant acid phosphatase 5b, may be advantageous for patients with advanced chronic kidney disease. Further studies of the use of BTMs in individuals with metabolic bone disease, coupled with the harmonization of commercial assays to provide equivalent results, will further enhance their clinical applications.
Keyword
Figure
Reference
-
1. Vasikaran S, Eastell R, Bruyère O, Foldes AJ, Garnero P, Griesmacher A, et al. 2011; Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 22:391–420. DOI: 10.1007/s00198-010-1501-1. PMID: 21184054.
Article2. Delmas PD, Eastell R, Garnero P, Seibel MJ, Stepan J. Committee of Scientific Advisors of the International Osteoporosis Foundation. 2000; The use of biochemical markers of bone turnover in osteoporosis. Osteoporos Int. 11(Suppl 6):S2–17. DOI: 10.1007/s001980070002. PMID: 11193237.
Article3. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. 2017; KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 7:1–59. DOI: 10.1016/j.kisu.2017.04.001. PMID: 30675420. PMCID: PMC6340919.4. Peach H, Compston JE, Vedi S, Horton LW. 1982; Value of plasma calcium, phosphate, and alkaline phosphatase measurements in the diagnosis of histological osteomalacia. J Clin Pathol. 35:625–30. DOI: 10.1136/jcp.35.6.625. PMID: 7085914. PMCID: PMC497738.
Article5. Ralston SH, Corral-Gudino L, Cooper C, Francis RM, Fraser WD, Gennari L, et al. 2019; Diagnosis and management of Paget's disease of bone in adults: a clinical guideline. J Bone Miner Res. 34:579–604. DOI: 10.1002/jbmr.3657. PMID: 30803025. PMCID: PMC6522384.
Article6. Alonso S, Ferrero E, Donat M, Martínez G, Vargas C, Hidalgo M, et al. 2012; The usefulness of high pre-operative levels of serum type I collagen bone markers for the prediction of changes in bone mineral density after parathyroidectomy. J Endocrinol Invest. 35:640–4.7. Yang L, Grey V. 2006; Pediatric reference intervals for bone markers. Clin Biochem. 39:561–8. DOI: 10.1016/j.clinbiochem.2005.11.015. PMID: 16423337.
Article8. Tobiume H, Kanzaki S, Hida S, Ono T, Moriwake T, Yamauchi S, et al. 1997; Serum bone alkaline phosphatase isoenzyme levels in normal children and children with growth hormone (GH) deficiency: a potential marker for bone formation and response to GH therapy. J Clin Endocrinol Metab. 82:2056–61. DOI: 10.1210/jc.82.7.2056.
Article9. Pi YZ, Wu XP, Liu SP, Luo XH, Cao XZ, Xie H, et al. 2006; Age-related changes in bone biochemical markers and their relationship with bone mineral density in normal Chinese women. J Bone Miner Metab. 24:380–5. DOI: 10.1007/s00774-006-0703-2. PMID: 16937270.
Article10. Szulc P, Seeman E, Delmas PD. 2000; Biochemical measurements of bone turnover in children and adolescents. Osteoporos Int. 11:281–94. DOI: 10.1007/s001980070116. PMID: 10928217.
Article11. Braga V, Gatti D, Rossini M, Colapietro F, Battaglia E, Viapiana O, et al. 2004; Bone turnover markers in patients with osteogenesis imperfecta. Bone. 34:1013–6. DOI: 10.1016/j.bone.2004.02.023. PMID: 15193547.
Article12. Chubb SAP, Vasikaran SD, Gillett MJ. 2023; Reference intervals for plasma β-CTX and P1NP in children: a systematic review and pooled estimates. Clin Biochem. 118:110582. DOI: 10.1016/j.clinbiochem.2023.05.001. PMID: 37187224.
Article13. Hoshino H, Kushida K, Takahashi M, Yamazaki K, Denda M, Atsumi K, et al. 2000; Changes in levels of biochemical markers and ultrasound indices of Os calcis across the menopausal transition. Osteoporos Int. 11:128–33. DOI: 10.1007/s001980050016. PMID: 10793870.
Article14. Heaney RP, Recker RR, Saville PD. 1978; Menopausal changes in bone remodeling. J Lab Clin Med. 92:964–70.15. Naylor K, Eastell R. 2012; Bone turnover markers: use in osteoporosis. Nat Rev Rheumatol. 8:379–89. DOI: 10.1038/nrrheum.2012.86. PMID: 22664836.
Article16. Morris HA, Eastell R, Jorgensen NR, Cavalier E, Vasikaran S, Chubb SAP, et al. 2017; Clinical usefulness of bone turnover marker concentrations in osteoporosis. Clin Chim Acta. 467:34–41. DOI: 10.1016/j.cca.2016.06.036. PMID: 27374301.
Article17. Cho DH, Chung JO, Chung MY, Cho JR, Chung DJ. 2020; Reference intervals for bone turnover markers in Korean healthy women. J Bone Metab. 27:43–52. DOI: 10.11005/jbm.2020.27.1.43. PMID: 32190608. PMCID: PMC7064366.
Article18. Yoo JI, Park AJ, Lim YK, Kweon OJ, Choi JH, Do JH, et al. 2018; Age-related reference intervals for total collagen-I-N-terminal propeptide in healthy Korean population. J Bone Metab. 25:235–41. DOI: 10.11005/jbm.2018.25.4.235. PMID: 30574468. PMCID: PMC6288608.
Article19. Hu WW, Zhang Z, He JW, Fu WZ, Wang C, Zhang H, et al. 2013; Establishing reference intervals for bone turnover markers in the healthy Shanghai population and the relationship with bone mineral density in postmenopausal women. Int J Endocrinol. 2013:513925. DOI: 10.1155/2013/513925. PMID: 23533403. PMCID: PMC3600195.
Article20. Li M, Li Y, Deng W, Zhang Z, Deng Z, Hu Y, et al. Chinese bone turnover marker study: reference ranges for C-terminal telopeptide of type I collagen and procollagen I N-terminal peptide by age and gender. PLoS One. 2014; 9:e103841. DOI: 10.1371/journal.pone.0103841. PMID: 25117452. PMCID: PMC4130521.
Article21. Sun LL, Cao RR, Wang JD, Zhang GL, Deng FY, Lei SF. 2023; Establishment of reference intervals for bone turnover markers in healthy Chinese older adults. Ann Hum Biol. 50:172–86. DOI: 10.1080/03014460.2023.2187456. PMID: 36882371.
Article22. Nishizawa Y, Miura M, Ichimura S, Inaba M, Imanishi Y, Shiraki M, et al. 2019; Executive summary of the Japan Osteoporosis Society Guide for the Use of Bone Turnover Markers in the Diagnosis and Treatment of Osteoporosis (2018 Edition). Clin Chim Acta. 498:101–107. DOI: 10.1016/j.cca.2019.08.012. PMID: 31425674.
Article23. Wu CH, Chang YF, Chen CH, Lewiecki EM, Wüster C, Reid I, et al. 2021; Consensus statement on the use of bone turnover markers for short-term monitoring of osteoporosis treatment in the Asia-Pacific region. J Clin Densitom. 24:3–13. DOI: 10.1016/j.jocd.2019.03.004. PMID: 31010789.
Article24. Park SY, Ahn SH, Yoo JI, Chung YJ, Jeon YK, Yoon BH, et al. 2019; Position statement on the use of bone turnover markers for osteoporosis treatment. J Bone Metab. 26:213–24. DOI: 10.11005/jbm.2019.26.4.213. PMID: 31832387. PMCID: PMC6901690.
Article25. Vasikaran SD, Miura M, Pikner R, Bhattoa HP, Cavalier E. IOF-IFCC Joint Committee on Bone Metabolism (C-BM). 2023; Practical considerations for the clinical application of bone turnover markers in osteoporosis. Calcif Tissue Int. 112:148–57. DOI: 10.1007/s00223-021-00930-4. PMID: 34846540.
Article26. Vasikaran SD, Chubb SAP. 2016; The use of biochemical markers of bone turnover in the clinical management of primary and secondary osteoporosis. Endocrine. 52:222–5. DOI: 10.1007/s12020-016-0900-2. PMID: 26906711.
Article27. Eastell R, Delmas PD. 2005; How to interpret surrogate markers of efficacy in osteoporosis. J Bone Miner Res. 20:1261–2. author reply 1263–4. DOI: 10.1359/JBMR.050217. PMID: 15940381.
Article28. Vasikaran SD, McCloskey EV, Kahn S, Kanis JA. 1994; Intra-individual variation in fasting urinary calcium- and hydroxyproline-creatinine ratios measured in metabolic bone clinic patients as both outpatients and inpatients. Ann Clin Biochem. 31:272–6. DOI: 10.1177/000456329403100310. PMID: 8067669.
Article29. Szulc P, Naylor K, Hoyle NR, Eastell R, Leary ET. National Bone Health Alliance Bone Turnover Marker Project. 2017; Use of CTX-I and PINP as bone turnover markers: National Bone Health Alliance recommendations to standardize sample handling and patient preparation to reduce pre-analytical variability. Osteoporos Int. 28:2541–56. DOI: 10.1007/s00198-017-4082-4. PMID: 28631236.
Article30. Kanis JA, Melton LJ, Christiansen C, Johnston CC, Khaltaev N. 1994; The diagnosis of osteoporosis. J Bone Miner Res. 9:1137–41. DOI: 10.1002/jbmr.5650090802. PMID: 7976495.
Article31. Chandran M, Bhadada SK, Ebeling PR, Gilchrist NL, Khan AH, Halbout P, et al. 2020; IQ driving QI: the Asia Pacific Consortium on Osteoporosis (APCO): an innovative and collaborative initiative to improve osteoporosis care in the Asia Pacific. Osteoporos Int. 31:2077–81. DOI: 10.1007/s00198-020-05495-w. PMID: 32561953. PMCID: PMC7560927.
Article32. Mithal A, Bansal B, Kyer CS, Ebeling P. The Asia-Pacific regional audit-epidemiology, costs, and burden of osteoporosis in India 2013: a report of International Osteoporosis Foundation. Indian J Endocrinol Metab. 2014; 18:449–54. DOI: 10.4103/2230-8210.137485. PMID: 25143898. PMCID: PMC4138897.
Article33. Garnero P, Hausherr E, Chapuy MC, Marcelli C, Grandjean H, Muller C, et al. 1996; Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res. 11:1531–8. DOI: 10.1002/jbmr.5650111021. PMID: 8889854.
Article34. Garnero P, Sornay-Rendu E, Claustrat B, Delmas PD. 2000; Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res. 15:1526–36. DOI: 10.1359/jbmr.2000.15.8.1526. PMID: 10934651.
Article35. McCloskey EV, Vasikaran S, Cooper C. FRAX (®) Position Development Conference Members. 2011; Official positions for FRAX® clinical regarding biochemical markers from Joint Official Positions Development Conference of the International Society for Clinical Densitometry and International Osteoporosis Foundation on FRAX®. J Clin Densitom. 14:220–2. DOI: 10.1016/j.jocd.2011.05.008. PMID: 21810528.36. Reginster JY, Collette J, Neuprez A, Zegels B, Deroisy R, Bruyere O. 2008; Role of biochemical markers of bone turnover as prognostic indicator of successful osteoporosis therapy. Bone. 42:832–6. DOI: 10.1016/j.bone.2008.01.021. PMID: 18316258.
Article37. Eastell R, Pigott T, Gossiel F, Naylor KE, Walsh JS, Peel NFA. 2018; Diagnosis of endocrine disease: bone turnover markers: are they clinically useful? Eur J Endocrinol. 178:R19–31. DOI: 10.1530/EJE-17-0585. PMID: 29046326.
Article38. Lorentzon M, Branco J, Brandi ML, Bruyère O, Chapurlat R, Cooper C, et al. 2019; Algorithm for the use of biochemical markers of bone turnover in the diagnosis, assessment and follow-up of treatment for osteoporosis. Adv Ther. 36:2811–24. DOI: 10.1007/s12325-019-01063-9. PMID: 31440982. PMCID: PMC6822833.
Article39. Meier C, Eastell R, Pierroz DD, Lane NE, Al-Daghri N, Suzuki A, et al. 2023; Biochemical markers of bone fragility in patients with diabetes. A narrative review by the IOF and the ECTS. J Clin Endocrinol Metab. dgad255. DOI: 10.1210/clinem/dgad255. PMCID: PMC10505554.40. Krege JH, Lane NE, Harris JM, Miller PD. 2014; PINP as a biological response marker during teriparatide treatment for osteoporosis. Osteoporos Int. 25:2159–71. DOI: 10.1007/s00198-014-2646-0. PMID: 24599274. PMCID: PMC4134485.
Article41. Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, et al. 2004; Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 350:1189–99. DOI: 10.1056/NEJMoa030897. PMID: 15028823.
Article42. Grey A, Bolland MJ, Horne A, Wattie D, House M, Gamble G, et al. 2012; Five years of anti-resorptive activity after a single dose of zoledronate - results from a randomized double-blind placebo-controlled trial. Bone. 50:1389–93. DOI: 10.1016/j.bone.2012.03.016. PMID: 22465268.
Article43. Adler RA, El-Hajj Fuleihan G, Bauer DC, Camacho PM, Clarke BL, Clines GA, et al. 2016; Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 31:16–35. DOI: 10.1002/jbmr.2708. PMID: 26350171. PMCID: PMC4906542.
Article44. Bauer DC, Schwartz A, Palermo L, Cauley J, Hochberg M, Santora A, et al. 2014; Fracture prediction after discontinuation of 4 to 5 years of alendronate therapy: the FLEX study. JAMA Intern Med. 174:1126–34. DOI: 10.1001/jamainternmed.2014.1232. PMID: 24798675. PMCID: PMC4409325.
Article45. Cosman F, Cauley JA, Eastell R, Boonen S, Palermo L, Reid IR, et al. 2014; Reassessment of fracture risk in women after 3 years of treatment with zoledronic acid: when is it reasonable to discontinue treatment? J Clin Endocrinol Metab. 99:4546–54. DOI: 10.1210/jc.2014-1971. PMID: 25215556.
Article46. Bone HG, Bolognese MA, Yuen CK, Kendler DL, Miller PD, Yang YC, et al. 2011; Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 96:972–80. DOI: 10.1210/jc.2010-1502. PMID: 21289258.
Article47. Zanchetta MB, Boailchuk J, Massari F, Silveira F, Bogado C, Zanchetta JR. 2018; Significant bone loss after stopping long-term denosumab treatment: a post FREEDOM study. Osteoporos Int. 29:41–7. DOI: 10.1007/s00198-017-4242-6. PMID: 28975362.
Article48. Tsourdi E, Zillikens MC, Meier C, Body JJ, Gonzalez Rodriguez E, Anastasilakis AD, et al. 2020; Fracture risk and management of discontinuation of denosumab therapy: a systematic review and position statement by ECTS. J Clin Endocrinol Metab. dgaa756. DOI: 10.1210/clinem/dgaa756. PMID: 33103722.
Article49. Alvarez L, Torregrosa JV, Peris P, Monegal A, Bedini JL, Martinez De Osaba MJ, et al. 2004; Effect of hemodialysis and renal failure on serum biochemical markers of bone turnover. J Bone Miner Metab. 22:254–9. DOI: 10.1007/s00774-003-0476-9. PMID: 15108068.
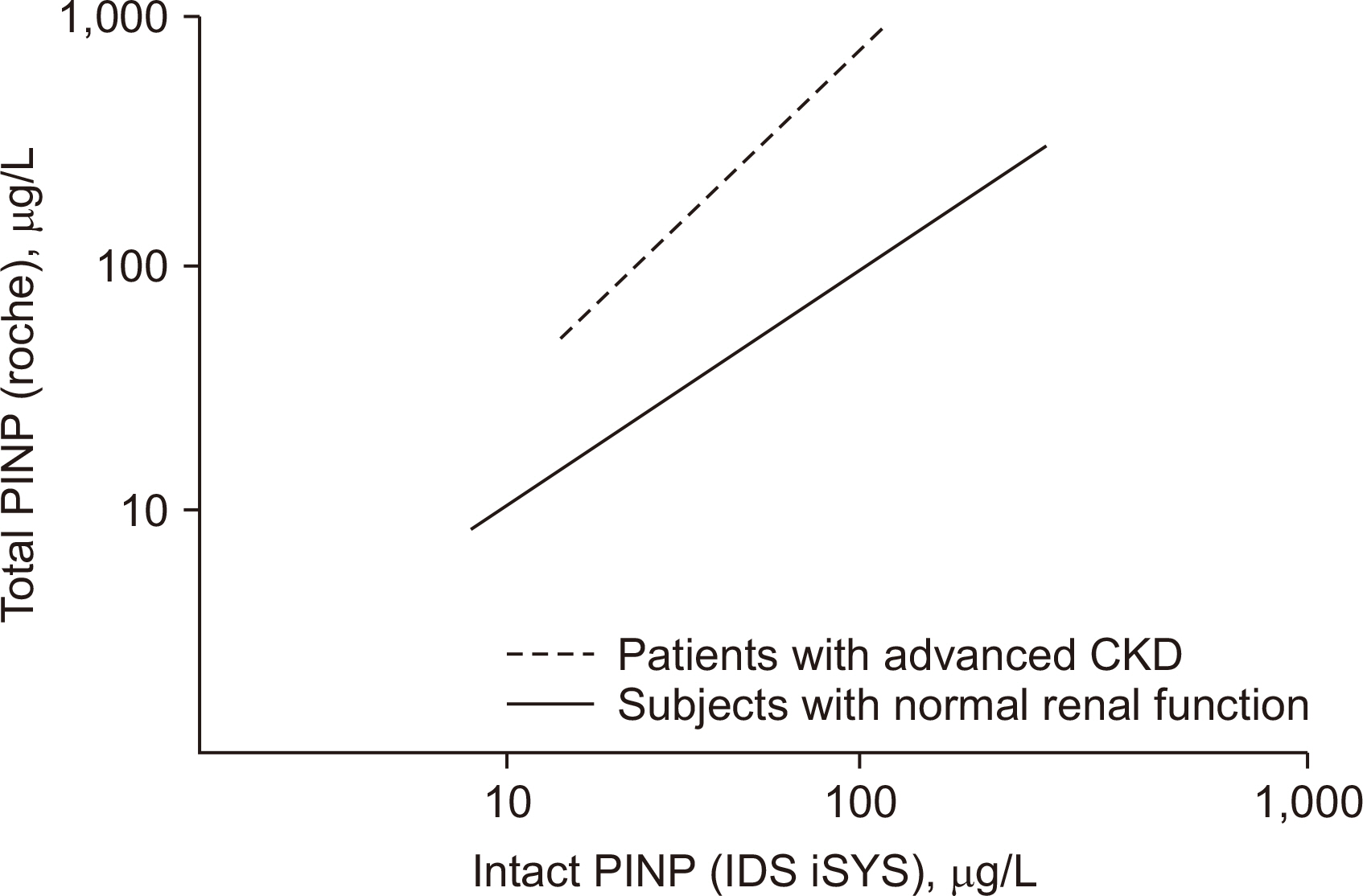
Article50. Tridimas A, Milan A, Marks E. 2021; Assessing bone formation in patients with chronic kidney disease using procollagen type I N-terminal propeptide (PINP): the choice of assay makes a difference. Ann Clin Biochem. 58:528–36. DOI: 10.1177/00045632211025567. PMID: 34096326.
Article51. Sprague SM, Bellorin-Font E, Jorgetti V, Carvalho AB, Malluche HH, Ferreira A, et al. 2016; Diagnostic accuracy of bone turnover markers and bone histology in patients with CKD treated by dialysis. Am J Kidney Dis. 67:559–66. DOI: 10.1053/j.ajkd.2015.06.023. PMID: 26321176.
Article52. Salam S, Gallagher O, Gossiel F, Paggiosi M, Khwaja A, Eastell R. 2018; Diagnostic accuracy of biomarkers and imaging for bone turnover in renal osteodystrophy. J Am Soc Nephrol. 29:1557–65. DOI: 10.1681/ASN.2017050584. PMID: 29555831. PMCID: PMC5967779.
Article53. Pimentel A, Ureña-Torres P, Zillikens MC, Bover J, Cohen-Solal M. 2017; Fractures in patients with CKD-diagnosis, treatment, and prevention: a review by members of the European Calcified Tissue Society and the European Renal Association of Nephrology Dialysis and Transplantation. Kidney Int. 92:1343–55. DOI: 10.1016/j.kint.2017.07.021. PMID: 28964571.
Article54. Schini M, Vilaca T, Gossiel F, Salam S, Eastell R. 2023; Bone turnover markers: basic biology to clinical applications. Endocr Rev. 44:417–73. DOI: 10.1210/endrev/bnac031. PMID: 36510335. PMCID: PMC10166271.
Article55. Bhattoa HP, Cavalier E, Eastell R, Heijboer AC, Jørgensen NR, Makris K, et al. 2021; Analytical considerations and plans to standardize or harmonize assays for the reference bone turnover markers PINP and β-CTX in blood. Clin Chim Acta. 515:16–20. DOI: 10.1016/j.cca.2020.12.023. PMID: 33382995. PMCID: PMC8033406.
Article