J Korean Med Sci.
2024 Feb;39(6):e55. 10.3346/jkms.2024.39.e55.
Pneumonia Prevalence Upon Chest Radiography According to Vaccination Status Among Patients Under 50 Years of Age With Coronavirus Disease 2019
- Affiliations
-
- 1Division of Pulmonology, Department of Internal Medicine, Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea
- 2Division of Infectious Diseases, Department of Internal Medicine, Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea
- 3Department of Radiology, Dongsan Hospital, Keimyung University College of Medicine, Daegu, Korea
- KMID: 2553306
- DOI: http://doi.org/10.3346/jkms.2024.39.e55
Abstract
- Background
Coronavirus disease 2019 (COVID-19) vaccination is effective in preventing the disease transmission and progression. However, the relatively mild disease course of the omicron variant and the decrease in antibodies over time after vaccination raise questions about the effectiveness of vaccination, especially in young people. We compared the prevalence of pneumonia and chest X-ray severity score according to vaccination status among patients < 50 years old with COVID-19.
Methods
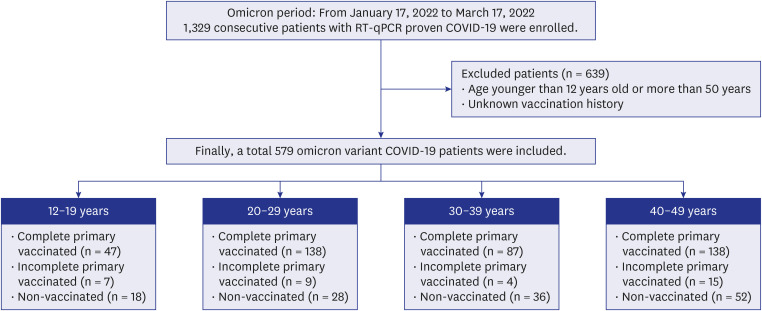
From January 17 to March 17, 2022, 579 patients with COVID-19, who were < 50 years old and had a known vaccination history in our institution, were all included in this study. All patients underwent initial chest radiography, and follow-up chest radiographs were obtained every two days until discharge. Pneumonia was scored from the radiographs using the Brixia scoring system. The scores of the six lung zones were added for a total score ranging from 0 to 18. Patients were divided into four groups according to 10-year age intervals. Differences between groups were analyzed using the χ2 or Fisher’s exact tests for categorical variables and the Kruskal–Wallis test or analysis of variance for continuous variables.
Results
Among patients aged 12–19 years, the prevalence of pneumonia did not differ depending on vaccination status (non-vaccinated vs. vaccinated, 1/47 [2.1%] vs. 1/18 [5.6%]; P = 0.577). Among patients in their 20s, the prevalence of pneumonia was significantly higher among nonvaccinated patients than among vaccinated patients (8/28, 28.6% vs. 7/138, 5.1%, P < 0.001), similar to patients in their 40s (32/52 [61.5%] vs. 18/138 [13.0%]; P < 0.001). The chest X-ray severity score was also significantly higher in non-vaccinated patients than that in vaccinated patients in their 20s to their 40s (P < 0.001), but not among patients aged 12–19 years (P = 0.678).
Conclusion
In patients aged 20–49 years, vaccinated patients had a significantly lower prevalence of pneumonia and chest X-ray severity score than non-vaccinated patients.
Figure
Reference
-
1. Daria S, Bhuiyan MA, Islam MR. Detection of highly muted coronavirus variant omicron (B.1.1.529) is triggering the alarm for South Asian countries: associated risk factors and preventive actions. J Med Virol. 2022; 94(4):1267–1268. PMID: 34862624.2. Lee JJ, Choe YJ, Jeong H, Kim M, Kim S, Yoo H, et al. Importation and transmission of SARS-CoV-2 B.1.1.529 (omicron) variant of concern in Korea, November 2021. J Korean Med Sci. 2021; 36(50):e346. PMID: 34962117.3. Sigal A. Milder disease with omicron: is it the virus or the pre-existing immunity? Nat Rev Immunol. 2022; 22(2):69–71. PMID: 35046570.4. Arbel R, Hammerman A, Sergienko R, Friger M, Peretz A, Netzer D, et al. BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med. 2021; 385(26):2413–2420. PMID: 34879190.5. Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021; 384(15):1412–1423. PMID: 33626250.6. Gilboa M, Mandelboim M, Indenbaum V, Lustig Y, Cohen C, Rahav G, et al. Early immunogenicity and safety of the third dose of BNT162b2 messenger RNA coronavirus disease 2019 vaccine among adults older than 60 years: real-world experience. J Infect Dis. 2022; 225(5):785–792. PMID: 34850049.7. Chenchula S, Karunakaran P, Sharma S, Chavan M. Current evidence on efficacy of COVID-19 booster dose vaccination against the omicron variant: a systematic review. J Med Virol. 2022; 94(7):2969–2976. PMID: 35246846.8. Centers for Disease Control and Prevention. Stay up to date with COVID-19 vaccines. Accessed June 30, 2023. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/stay-up-to-date.html .9. Troiano G, Nardi A. Vaccine hesitancy in the era of COVID-19. Public Health. 2021; 194:245–251. PMID: 33965796.10. Buchan SA, Chung H, Brown KA, Austin PC, Fell DB, Gubbay JB, et al. Estimated effectiveness of COVID-19 vaccines against omicron or delta symptomatic infection and severe outcomes. JAMA Netw Open. 2022; 5(9):e2232760. PMID: 36136332.11. Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022; 386(16):1532–1546. PMID: 35249272.12. Nham E, Song JY, Noh JY, Cheong HJ, Kim WJ. COVID-19 vaccination in Korea: past, present, and the way forward. J Korean Med Sci. 2022; 37(47):e351. PMID: 36472087.13. Kelly JD, Leonard S, Hoggatt KJ, Boscardin WJ, Lum EN, Moss-Vazquez TA, et al. Incidence of severe COVID-19 illness following vaccination and booster with BNT162b2, mRNA-1273, and Ad26.COV2.S vaccines. JAMA. 2022; 328(14):1427–1437. PMID: 36156706.14. Borghesi A, Zigliani A, Masciullo R, Golemi S, Maculotti P, Farina D, et al. Radiographic severity index in COVID-19 pneumonia: relationship to age and sex in 783 Italian patients. Radiol Med (Torino). 2020; 125(5):461–464. PMID: 32358691.15. Centers for Disease Control and Prevention. People with certain medical conditions. Accessed September 20, 2023. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html .16. Booth A, Reed AB, Ponzo S, Yassaee A, Aral M, Plans D, et al. Population risk factors for severe disease and mortality in COVID-19: a global systematic review and meta-analysis. PLoS One. 2021; 16(3):e0247461. PMID: 33661992.17. Wu N, Joyal-Desmarais K, Ribeiro PA, Vieira AM, Stojanovic J, Sanuade C, et al. Long-term effectiveness of COVID-19 vaccines against infections, hospitalisations, and mortality in adults: findings from a rapid living systematic evidence synthesis and meta-analysis up to December, 2022. Lancet Respir Med. 2023; 11(5):439–452. PMID: 36780914.18. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020; 324(8):782–793. PMID: 32648899.19. Gibson PG, Qin L, Puah SH. COVID-19 acute respiratory distress syndrome (ARDS): clinical features and differences from typical pre-COVID-19 ARDS. Med J Aust. 2020; 213(2):54–56.e1. PMID: 32572965.20. Li M, Liu Q, Wu D, Tang L, Wang X, Yan T, et al. Association of COVID-19 vaccination and clinical severity of patients infected with delta or omicron variants - China, May 21, 2021-February 28, 2022. China CDC Wkly. 2022; 4(14):293–297. PMID: 35433093.21. Lauring AS, Tenforde MW, Chappell JD, Gaglani M, Ginde AA, McNeal T, et al. Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ. 2022; 376:e069761. PMID: 35264324.22. Melendi SE, Pérez MM, Salas CE, Haedo MF, Xavier FB, Saltos Navarrete JD, et al. COVID-19 with and without pneumonia: clinical outcomes in the internal medicine ward. Medicina (B Aires). 2020; 80(Suppl 6):56–64. PMID: 33481734.23. Florentino PT, Millington T, Cerqueira-Silva T, Robertson C, de Araújo Oliveira V, Júnior JB, et al. Vaccine effectiveness of two-dose BNT162b2 against symptomatic and severe COVID-19 among adolescents in Brazil and Scotland over time: a test-negative case-control study. Lancet Infect Dis. 2022; 22(11):1577–1586. PMID: 35952702.24. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020; 383(27):2603–2615. PMID: 33301246.25. Oster ME, Shay DK, Su JR, Gee J, Creech CB, Broder KR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022; 327(4):331–340. PMID: 35076665.26. World Health Organization. COVID-19 subcommittee of the WHO Global Advisory Committee on Vaccine Safety (GACVS): updated statement regarding myocarditis and pericarditis reported with COVID-19 mRNA vaccines. Updated 2021. Accessed June 30, 2023. https://www.who.int/news/item/27-10-2021-gacvs-statement-myocarditis-pericarditis-covid-19-mrna-vaccines-updated .27. World Health Organization. Interim statement on COVID-19 vaccination for children. Updated 2022. Accessed December 11, 2022. https://www.who.int/news/item/11-08-2022-interim-statement-on-covid-19-vaccination-for-children .28. Centers for Disease Control and Prevention. COVID-19 vaccine safety in children and teens. Updated 2023. Accessed June 30, 2023. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/vaccine-safety-children-teens.html .29. Block JP, Boehmer TK, Forrest CB, Carton TW, Lee GM, Ajani UA, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination - PCORnet, United States, January 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022; 71(14):517–523. PMID: 35389977.30. Ministry of Health and Welfare. COVID-19 occurrence status. Accessed August 29, 2023. https://ncov.kdca.go.kr/bdBoardList_Real.do .
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The First Case of 2019 Novel Coronavirus Pneumonia Imported into Korea from Wuhan, China: Implication for Infection Prevention and Control Measures
- Clinical and Radiologic Findings of COVID-19 Pneumonia: South Korean Experience from Three Cases
- Clinical and Radiological Findings of Coronavirus Disease 2019 Pneumonia: 51 Adult Patients from a Single Center in Daegu, South Korea
- Hemopneumothorax as an Unusual and Delayed Complication of Coronavirus Disease 2019 Pneumonia: A Case Report
- Mediastinal Emphysema, Giant Bulla, and PneumothoraxDeveloped during the Course of COVID-19 Pneumonia