Ann Lab Med.
2023 Nov;43(6):565-573. 10.3343/alm.2023.43.6.565.
Effect of Two Cystatin C Reagents and Four Equations on Glomerular Filtration Rate Estimations After Standardization
- Affiliations
-
- 1Department of Laboratory Medicine, School of Medicine, Wonkwang University, Iksan, Korea
- 2Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 3Division of Nephrology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 4Department of Medical Device Management and Research, Samsung Advanced Institute of Health Science and Technology, Sungkyunkwan University, Seoul, Korea
- KMID: 2552028
- DOI: http://doi.org/10.3343/alm.2023.43.6.565
Abstract
- Background
Serum cystatin C (cysC), which is less affected by sex, race, and muscle mass than creatinine, is a useful biomarker of the estimated glomerular filtration rate (eGFR). The standardization of cysC measurements remains controversial, although a certified reference material (ERM-DA471/IFCC) is available. Moreover, the effect of combinations of cysC reagents and equations for eGFR is unclear.
Methods
We conducted a simulation analysis of cysC measured using two reagents standardized against ERM-DA471/IFCC—Gentian cystatin C immunoassay (Gentiancys; GentianAS, Moss, Norway) and Roche Tina-quant Cystatin C Gen.2 (Rochecys; Roche, Mannheim, Germany)—on a Cobas c702 system (Roche) and eGFR generated by eight combinations of four equations: 2012 cystatin C-based Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPIcys); the Caucasian, Asian, pediatric, and adult equation (CAPAeq); full age spectrum equation (FASeq); and 2023 cystatin C-based European Kidney Function Consortium equation (EKFCcys).
Results
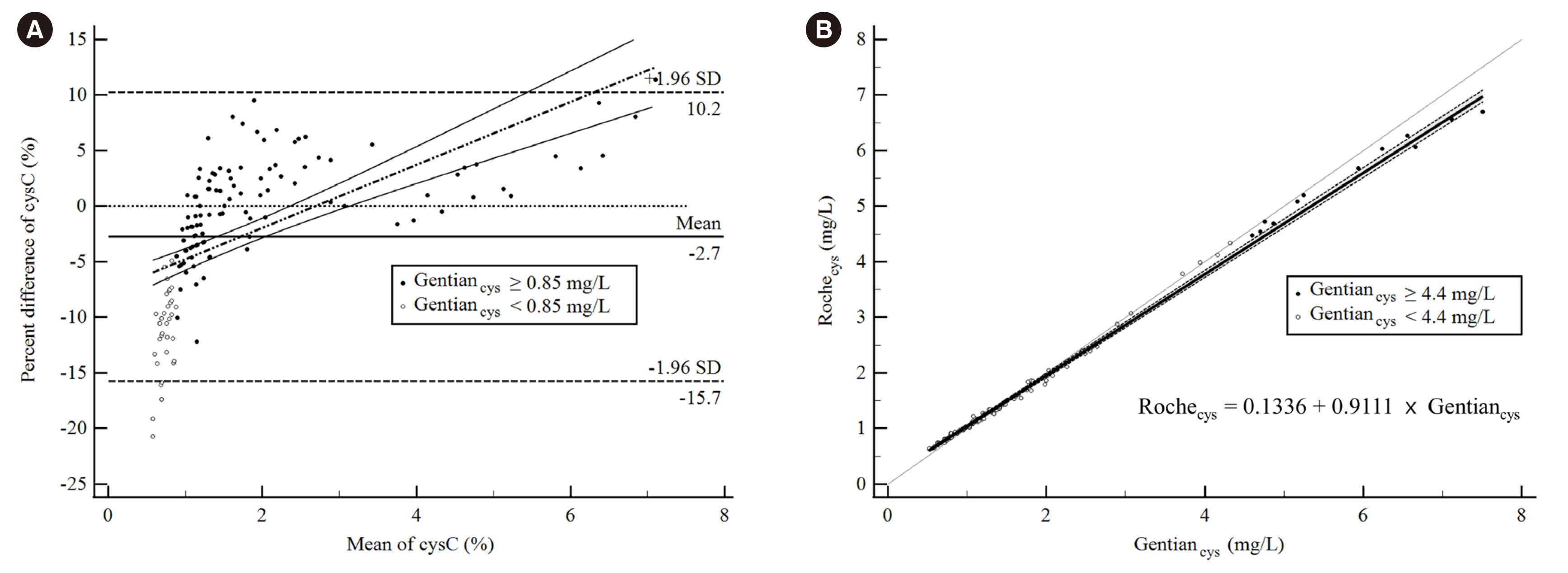
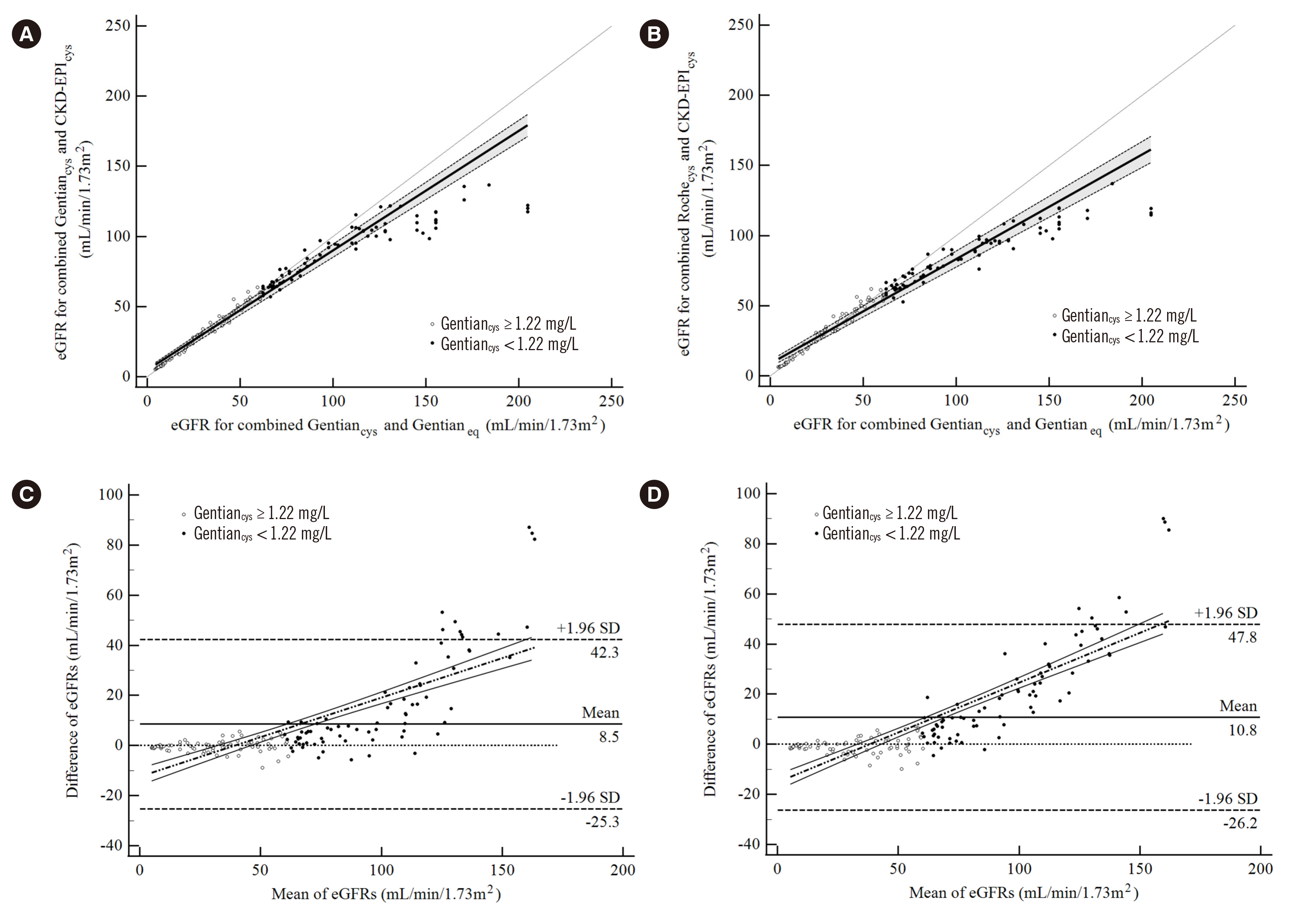
A total of 148 participants (mean age, 60.5±14.5 years; 43% female) were enrolled. The mean cysC was 1.72±1.44 mg/L for Gentiancys and 1.71±1.35 mg/L for Rochecys. Regression analysis showed concordance between the reagents within 0.85–4.40 mg/L when using ±7.61% total allowable error. Lin’s concordance correlation coefficient of eGFR, by combining the measuring system and equation, varied from 0.73 to 1.00.
Conclusions
The equivalence of cysC values at low concentrations (<0.85 mg/L) between the two reagents was unsatisfactory. Results obtained with different measurement systems could lead to larger differences in eGFR varying with the combination.
Keyword
Figure
Reference
-
1. Grubb A, Löfberg H. 1982; Human gamma-trace, a basic microprotein: amino acid sequence and presence in the adenohypophysis. Proc Natl Acad Sci U S A. 79:3024–7. DOI: 10.1073/pnas.79.9.3024. PMID: 6283552. PMCID: PMC346341.
Article2. Grubb A. 1992; Diagnostic value of analysis of cystatin C and protein HC in biological fluids. Clin Nephrol. 38(S1):S20–7.3. Filler G, Bökenkamp A, Hofmann W, Le Bricon T, Martínez-Brú C, Grubb A. 2005; Cystatin C as a marker of GFR-history, indications, and future research. Clin Biochem. 38:1–8. DOI: 10.1016/j.clinbiochem.2004.09.025. PMID: 15607309.
Article4. Knight EL, Verhave JC, Spiegelman D, Hillege HL, de Zeeuw D, Curhan GC, et al. 2004; Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 65:1416–21. DOI: 10.1111/j.1523-1755.2004.00517.x. PMID: 15086483.
Article5. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Working Group. 2013; KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 3:1–150.6. Ravn B, Prowle JR, Mårtensson J, Martling CR, Bell M. 2017; Superiority of serum cystatin C over creatinine in prediction of long-term prognosis at discharge from ICU. Crit Care Med. 45:e932–40. DOI: 10.1097/CCM.0000000000002537. PMID: 28614196.
Article7. Safdar OY, Shalaby M, Khathlan N, Elattal B, Bin Joubah M, Bukahri E, et al. 2016; Serum cystatin is a useful marker for the diagnosis of acute kidney injury in critically ill children: prospective cohort study. BMC Nephrol. 17:130. DOI: 10.1186/s12882-016-0346-z. PMID: 27624749. PMCID: PMC5022154.
Article8. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. 2012; Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 367:20–9. DOI: 10.1056/NEJMoa1114248. PMID: 22762315. PMCID: PMC4398023.
Article9. Larsson A, Hansson LO, Flodin M, Katz R, Shlipak MG. 2011; Calibration of the Siemens cystatin C immunoassay has changed over time. Clin Chem. 57:777–8. DOI: 10.1373/clinchem.2010.159848. PMID: 21364028.
Article10. Bargnoux AS, Piéroni L, Cristol JP, Kuster N, Delanaye P, Carlier MC, et al. 2017; Multicenter evaluation of cystatin C measurement after assay standardization. Clin Chem. 63:833–41. DOI: 10.1373/clinchem.2016.264325. PMID: 28188233.
Article11. Eckfeldt JH, Karger AB, Miller WG, Rynders GP, Inker LA. 2015; Performance in measurement of serum cystatin C by laboratories participating in the College of American Pathologists 2014 CYS survey. Arch Pathol Lab Med. 139:888–93. DOI: 10.5858/arpa.2014-0427-CP. PMID: 25884370.
Article12. Flodin M, Hansson LO, Larsson A. 2006; Variations in assay protocol for the Dako cystatin C method may change patient results by 50% without changing the results for controls. Clin Chem Lab Med. 44:1481–5. DOI: 10.1515/CCLM.2006.271. PMID: 17163826.
Article13. Bargnoux AS, Kuster N, Delatour V, Delanaye P, González-Antuña A, Cristol JP, et al. 2016; Reference method and reference material are necessary tools to reveal the variability of cystatin C assays. Arch Pathol Lab Med. 140:117–8. DOI: 10.5858/arpa.2015-0198-LE. PMID: 26910214.
Article14. Delanaye P, Pieroni L, Abshoff C, Lutteri L, Chapelle JP, Krzesinski JM, et al. 2008; Analytical study of three cystatin C assays and their impact on cystatin C-based GFR-prediction equations. Clin Chim Acta. 398:118–24. DOI: 10.1016/j.cca.2008.09.001. PMID: 18805407.
Article15. White CA, Rule AD, Collier CP, Akbari A, Lieske JC, Lepage N, et al. 2011; The impact of interlaboratory differences in cystatin C assay measurement on glomerular filtration rate estimation. Clin J Am Soc Nephrol. 6:2150–6. DOI: 10.2215/CJN.00130111. PMID: 21799146. PMCID: PMC3358990.
Article16. Inker LA, Eckfeldt J, Levey AS, Leiendecker-Foster C, Rynders G, Manzi J, et al. 2011; Expressing the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) cystatin C equations for estimating GFR with standardized serum cystatin C values. Am J Kidney Dis. 58:682–4. DOI: 10.1053/j.ajkd.2011.05.019. PMID: 21855190. PMCID: PMC4421875.
Article17. Grubb A, Horio M, Hansson LO, Björk J, Nyman U, Flodin M, et al. 2014; Generation of a new cystatin C-based estimating equation for glomerular filtration rate by use of 7 assays standardized to the international calibrator. Clin Chem. 60:974–86. DOI: 10.1373/clinchem.2013.220707. PMID: 24829272.
Article18. Pottel H, Delanaye P, Schaeffner E, Dubourg L, Eriksen BO, Melsom T, et al. 2017; Estimating glomerular filtration rate for the full age spectrum from serum creatinine and cystatin C. Nephrol Dial Transplant. 32:497–507. DOI: 10.1093/ndt/gfw425. PMID: 28089986. PMCID: PMC5837496.
Article19. Pottel H, Björk J, Rule AD, Ebert N, Eriksen BO, Dubourg L, et al. 2023; Cystatin C-based equation to estimate GFR without the inclusion of race and sex. N Engl J Med. 388:333–43. DOI: 10.1056/NEJMoa2203769. PMID: 36720134.
Article20. Ebert N, Delanaye P, Shlipak M, Jakob O, Martus P, Bartel J, et al. 2016; Cystatin C standardization decreases assay variation and improves assessment of glomerular filtration rate. Clin Chim Acta. 456:115–21. DOI: 10.1016/j.cca.2016.03.002. PMID: 26947968.
Article21. Flodin M, Jonsson AS, Hansson LO, Danielsson LA, Larsson A. 2007; Evaluation of Gentian cystatin C reagent on Abbott Ci8200 and calculation of glomerular filtration rate expressed in mL/min/1.73 m(2) from the cystatin C values in mg/L. Scand J Clin Lab Invest. 67:560–7. DOI: 10.1080/00365510601187773. PMID: 17763193.22. Westgard Q. Desirable Biological Variation Database specifications. https://www.westgard.com/biodatabase1.htm. Updated on May 2023.23. Lin L, Torbeck LD. 1998; Coefficient of accuracy and concordance correlation coefficient: new statistics for methods comparison. PDA J Pharm Sci Technol. 52:55–9.24. Grubb A, Nyman U, Björk J, Lindström V, Rippe B, Sterner G, et al. 2005; Simple cystatin C-based prediction equations for glomerular filtration rate compared with the modification of diet in renal disease prediction equation for adults and the Schwartz and the Counahan-Barratt prediction equations for children. Clin Chem. 51:1420–31. DOI: 10.1373/clinchem.2005.051557. PMID: 15961546.
Article25. Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. 2004; Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 141:929–37. DOI: 10.7326/0003-4819-141-12-200412210-00009. PMID: 15611490.
Article26. Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, et al. 2008; Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 51:395–406. DOI: 10.1053/j.ajkd.2007.11.018. PMID: 18295055. PMCID: PMC2390827.
Article27. Larsson A, Malm J, Grubb A, Hansson LO. 2004; Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scand J Clin Lab Invest. 64:25–30. DOI: 10.1080/00365510410003723. PMID: 15025426.
Article28. Tidman M, Sjöström P, Jones I. 2008; A comparison of GFR estimating formulae based upon s-cystatin C and s-creatinine and a combination of the two. Nephrol Dial Transplant. 23:154–60. DOI: 10.1093/ndt/gfm661. PMID: 17911090.
Article29. Grubb A, Blirup-Jensen S, Lindström V, Schmidt C, Althaus H, Zegers I, et al. 2010; First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin Chem Lab Med. 48:1619–21. DOI: 10.1515/CCLM.2010.318. PMID: 21034257.
Article30. Shlipak MG, Mattes MD, Peralta CA. 2013; Update on cystatin C: incorporation into clinical practice. Am J Kidney Dis. 62:595–603. DOI: 10.1053/j.ajkd.2013.03.027. PMID: 23701892. PMCID: PMC3755100.
Article31. Schwartz GJ, Cox C, Seegmiller JC, Maier PS, DiManno D, Furth SL, et al. 2020; Recalibration of cystatin C using standardized material in Siemens nephelometers. Pediatr Nephrol. 35:279–85. DOI: 10.1007/s00467-019-04389-2. PMID: 31680199. PMCID: PMC7249730.
Article32. Donadio C, Kanaki A, Caprio F, Donadio E, Tognotti D, Olivieri L. 2012; Prediction of glomerular filtration rate from serum concentration of cystatin C: comparison of two analytical methods. Nephrol Dial Transplant. 27:2826–38. DOI: 10.1093/ndt/gfs010. PMID: 22422869.
Article33. Benoit SW, Kathman T, Patel J, Stegman M, Cobb C, Hoehn J, et al. 2021; GFR estimation after cystatin C reference material change. Kidney Int Rep. 6:429–36. DOI: 10.1016/j.ekir.2020.11.028. PMID: 33615068. PMCID: PMC7879112.
Article34. Erlandsen EJ, Randers E. 2018; Reference intervals for plasma cystatin C and plasma creatinine in adults using methods traceable to international calibrators and reference methods. J Clin Lab Anal. 32:e22433. DOI: 10.1002/jcla.22433. PMID: 29573343. PMCID: PMC6817201.
Article35. Ataei N, Bazargani B, Ameli S, Madani A, Javadilarijani F, Moghtaderi M, et al. 2014; Early detection of acute kidney injury by serum cystatin C in critically ill children. Pediatr Nephrol. 29:133–8. DOI: 10.1007/s00467-013-2586-5. PMID: 23989306.
Article36. Murty MS, Sharma UK, Pandey VB, Kankare SB. 2013; Serum cystatin C as a marker of renal function in detection of early acute kidney injury. Indian J Nephrol. 23:180–3. DOI: 10.4103/0971-4065.111840. PMID: 23814415. PMCID: PMC3692142.
Article37. Rule AD, Rodeheffer RJ, Larson TS, Burnett JC, Cosio FG, Turner ST, et al. 2006; Limitations of estimating glomerular filtration rate from serum creatinine in the general population. Mayo Clin Proc. 81:1427–34. DOI: 10.4065/81.11.1427. PMID: 17120397.
Article38. Zhang Q, Cai Z, Lin H, Han L, Yan J, Wang J, et al. 2021; Expression, purification and identification of isotope-labeled recombinant cystatin C protein in Escheichia coli intended for absolute quantification using isotope dilution mass spectrometry. Protein Expr Purif. 178:105785. DOI: 10.1016/j.pep.2020.105785. PMID: 33152458.
Article39. Ji H, Wang J, Ju S, Cong H, Wang X, Su J, et al. 2017; Quantification of cystatin-C in human serum by stable isotope dilution liquid chromatography electrospray ionization tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 1059:49–55. DOI: 10.1016/j.jchromb.2017.04.007. PMID: 28578261.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Value of Serum Concentration of Cystatin C as a Marker for Glomerular Filtration Rate in Children and Adolescents
- Comparison of glomerular filtration rates calculated by different serum cystatin C-based equations in patients with chronic kidney disease
- Interpretation of Estimated Glomerular Filtration Rate
- Exploring Renal Function Assessment: Creatinine, Cystatin C, and Estimated Glomerular Filtration Rate Focused on the European Kidney Function Consortium Equation
- Cystatin C as a Marker for Early Renal Impairment