Int J Stem Cells.
2024 Feb;17(1):91-98. 10.15283/ijsc23043.
Mimicking the Human Articular Joint with In Vitro Model of Neurons-Synoviocytes Co-Culture
- Affiliations
-
- 1Department of Neurochemistry, Maj Institute of Pharmacology, Polish Academy of Sciences, Cracow, Poland
- 2Laboratory of Pharmacogenomics, Department of Molecular Pharmacology, Maj Institute of Pharmacology, Polish Academy of Sciences, Cracow, Poland
- KMID: 2552023
- DOI: http://doi.org/10.15283/ijsc23043
Abstract
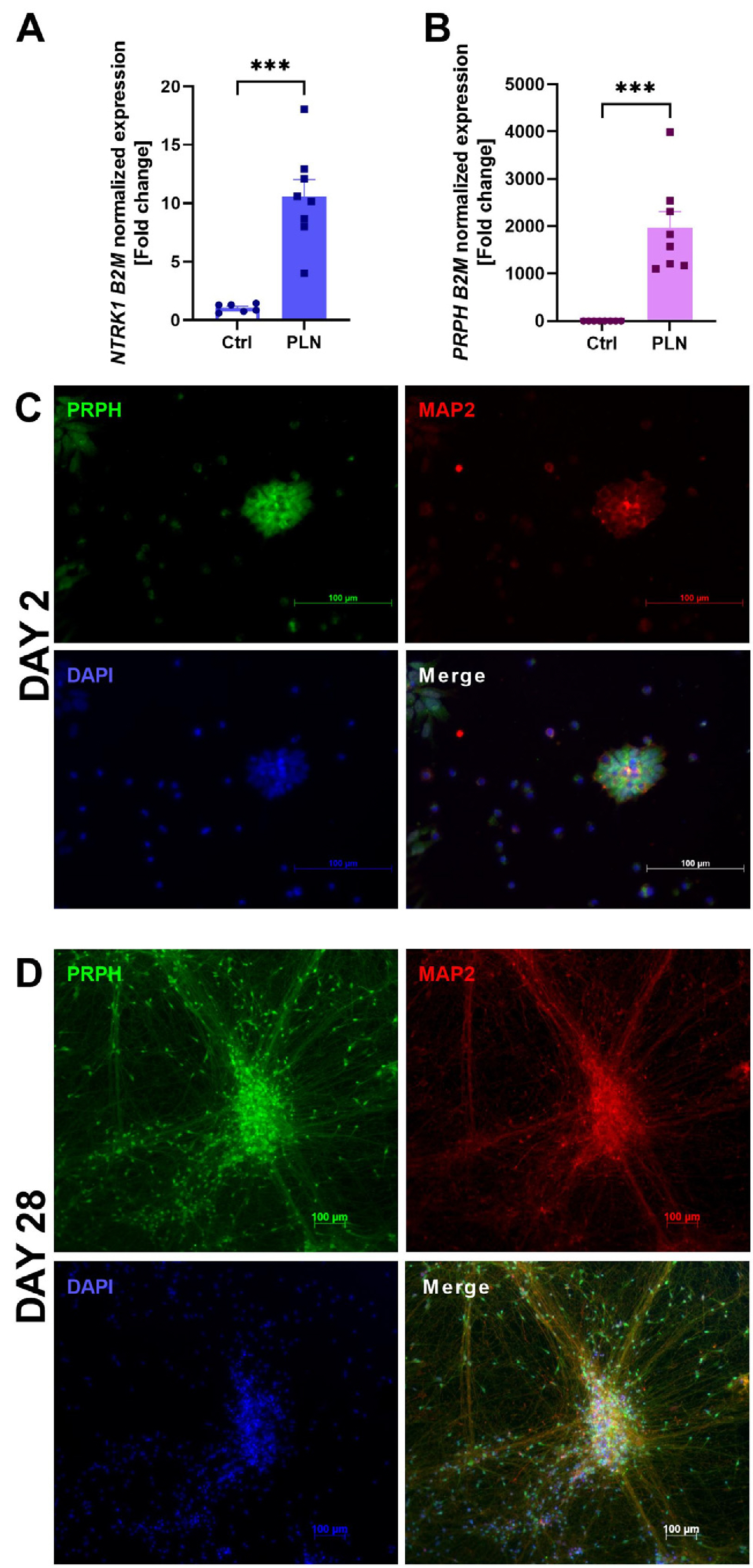
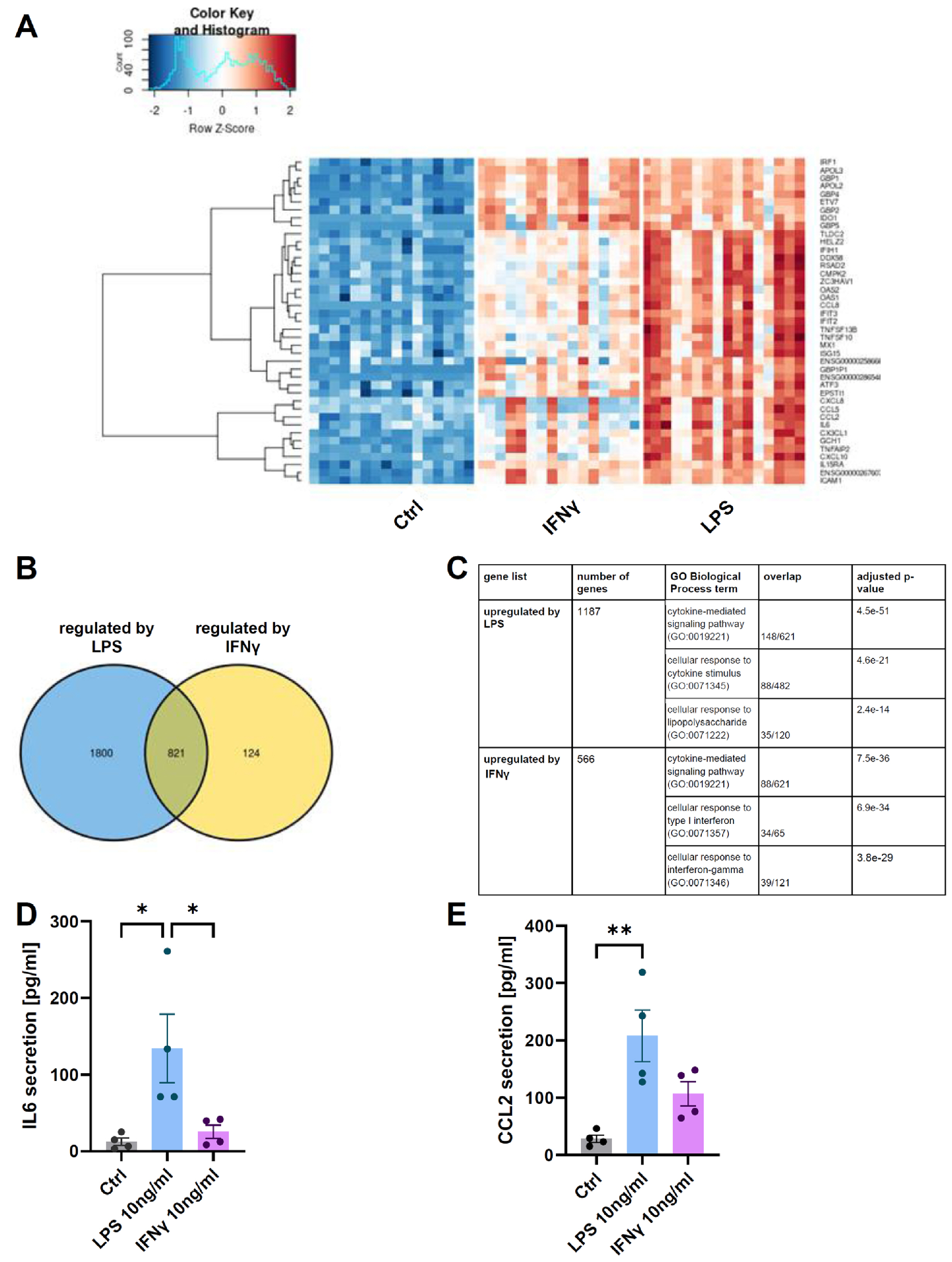
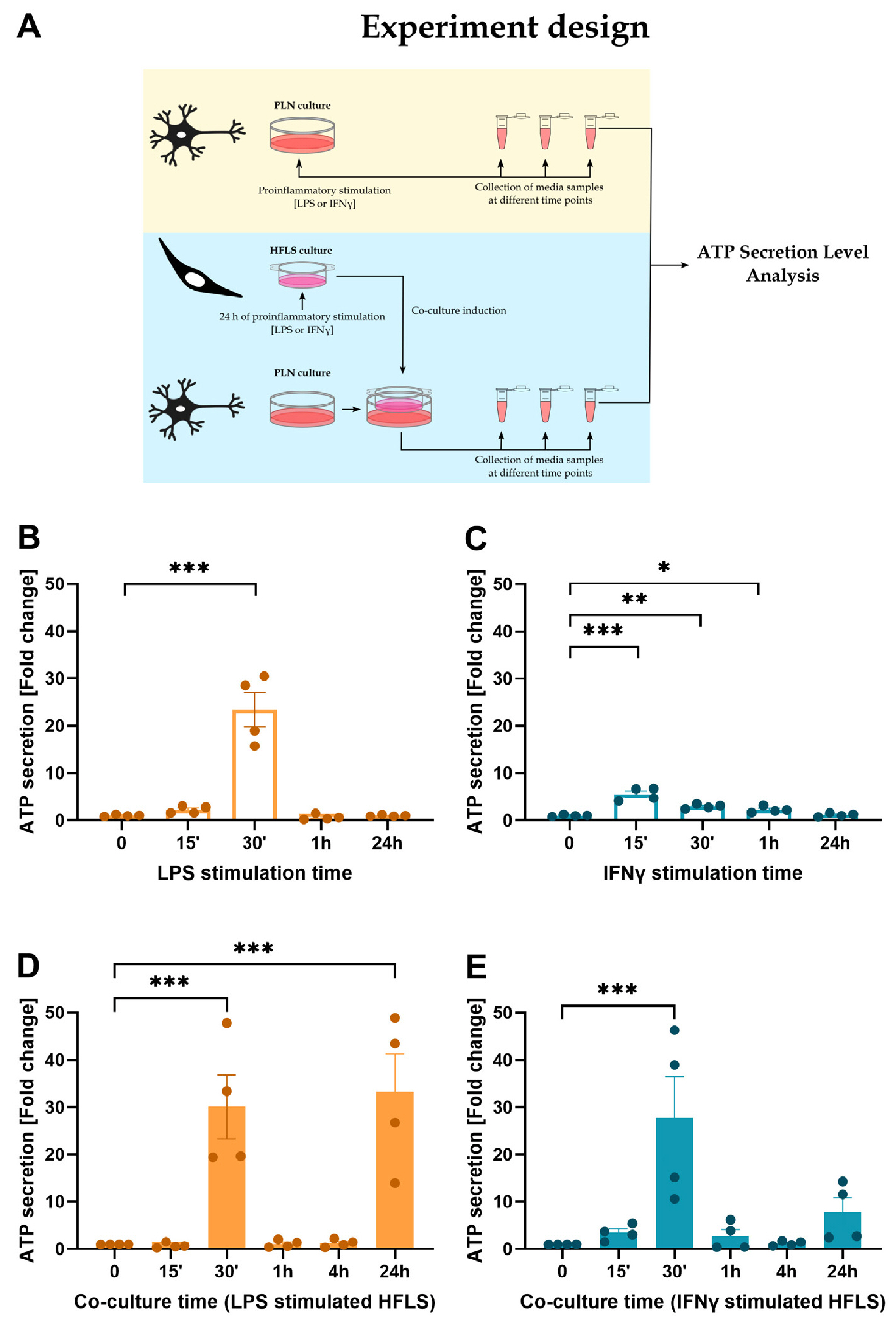
- The development of In Vitro models is essential in modern science due to the need for experiments using human material and the reduction in the number of laboratory animals. The complexity of the interactions that occur in living organisms requires improvements in the monolayer cultures. In the work presented here, neuroepithelial stem (NES) cells were differentiated into peripheral-like neurons (PLN) and the phenotype of the cells was confirmed at the genetic and protein levels. Then RNA-seq method was used to investigate how stimulation with pro-inflammatory factors such as LPS and IFNγ affects the expression of genes involved in the immune response in human fibroblast-like synoviocytes (HFLS). HFLS were then cultured on semi-permeable membrane inserts, and after 24 hours of pro-inflammatory stimulation, the levels of cytokines secretion into the medium were checked. Inserts with stimulated HFLS were introduced into the PLN culture, and by measuring secreted ATP, an increase in cell activity was found in the system. The method used mimics the condition that occurs in the joint during inflammation, as observed in the development of diseases such as rheumatoid arthritis (RA) or osteoarthritis (OA). In addition, the system used can be easily modified to simulate the interaction of peripheral neurons with other cell types.
Figure
Reference
-
References
1. de Sousa EB, Casado PL, Moura Neto V, Duarte ME, Aguiar DP. 2014; Synovial fluid and synovial membrane mesenchymal stem cells: latest discoveries and therapeutic pers-pectives. Stem Cell Res Ther. 5:112. DOI: 10.1186/scrt501. PMID: 25688673. PMCID: PMC4339206.
Article2. Grässel SG. 2014; The role of peripheral nerve fibers and their neurotransmitters in cartilage and bone physiology and pathophysiology. Arthritis Res Ther. 16:485. DOI: 10.1186/s13075-014-0485-1. PMID: 25789373. PMCID: PMC4395972.
Article3. Vasconcelos DP, Jabangwe C, Lamghari M, Alves CJ. 2022; The neuroimmune interplay in joint pain: the role of macro-phages. Front Immunol. 13:812962. DOI: 10.3389/fimmu.2022.812962. PMID: 35355986. PMCID: PMC8959978. PMID: 6a433392c87d4125a0c9fabe97d51257.
Article4. Cope PJ, Ourradi K, Li Y, Sharif M. 2019; Models of osteoarthritis: the good, the bad and the promising. Osteoarthritis Cartilage. 27:230–239. DOI: 10.1016/j.joca.2018.09.016. PMID: 30391394. PMCID: PMC6350005.
Article5. Roqué PJ, Costa LG. 2017; Co-culture of neurons and microglia. Curr Protoc Toxicol. 74:11.24.1–11.24.17. DOI: 10.1002/cptx.32. PMID: 29117434. PMCID: PMC5774987.
Article6. Zhao H, Sun P, Fan T, Yang X, Zheng T, Sun C. 2019; The effect of glutamate-induced excitotoxicity on DNA methylation in astrocytes in a new in vitro neuron-astrocyte-endothelium co-culture system. Biochem Biophys Res Commun. 508:1209–1214. DOI: 10.1016/j.bbrc.2018.12.058. PMID: 30558794.
Article7. Marcotti A, Miralles A, Dominguez E, et al. 2018; Joint nociceptor nerve activity and pain in an animal model of acute gout and its modulation by intra-articular hyaluronan. Pain. 159:739–748. DOI: 10.1097/j.pain.0000000000001137. PMID: 29319609. PMCID: PMC5895116.
Article8. Calvo-Garrido J, Winn D, Maffezzini C, et al. 2021; Protocol for the derivation, culturing, and differentiation of human iPS-cell-derived neuroepithelial stem cells to study neural differentiation in vitro. STAR Protoc. 2:100528. DOI: 10.1016/j.xpro.2021.100528. PMID: 34027486. PMCID: PMC8121988.9. Alshawaf AJ, Viventi S, Qiu W, et al. 2018; Phenotypic and functional characterization of peripheral sensory neurons deri-ved from human embryonic stem cells. Sci Rep. 8:603. DOI: 10.1038/s41598-017-19093-0. PMID: 29330377. PMCID: PMC5766621.
Article10. Lowin T, Zhu W, Dettmer-Wilde K, Straub RH. 2012; Cortisol-mediated adhesion of synovial fibroblasts is dependent on the degradation of anandamide and activation of the endocannabinoid system. Arthritis Rheum. 64:3867–3876. DOI: 10.1002/art.37684. PMID: 22933357.
Article11. Chwastek J, Kędziora M, Borczyk M, Korostyński M, Starowicz K. 2022; Inflammation-driven secretion potential is upregulated in osteoarthritic fibroblast-like synoviocytes. Int J Mol Sci. 23:11817. DOI: 10.3390/ijms231911817. PMID: 36233118. PMCID: PMC9570304. PMID: 99026a53073447d697ab6085db5d4ccd.
Article12. Xie Z, Bailey A, Kuleshov MV, et al. 2021; Gene set knowledge discovery with Enrichr. Curr Protoc. 1:e90. DOI: 10.1002/cpz1.90. PMID: 33780170. PMCID: PMC8152575.
Article13. Bufalo MC, Almeida MES, Jensen JR, et al. 2022; Human sensory neuron-like cells and glycated collagen matrix as a model for the screening of analgesic compounds. Cells. 11:247. DOI: 10.3390/cells11020247. PMID: 35053363. PMCID: PMC8773477. PMID: 6964b382dee845679af68cc0f5b3bbee.
Article14. Hui AY, McCarty WJ, Masuda K, Firestein GS, Sah RL. 2012; A systems biology approach to synovial joint lubrication in health, injury, and disease. Wiley Interdiscip Rev Syst Biol Med. 4:15–37. DOI: 10.1002/wsbm.157. PMID: 21826801. PMCID: PMC3593048.
Article15. Zhao J, Guo S, Schrodi SJ, He D. 2021; Molecular and cellular heterogeneity in rheumatoid arthritis: mechanisms and cli-nical implications. Front Immunol. 12:790122. DOI: 10.3389/fimmu.2021.790122. PMID: 34899757. PMCID: PMC8660630. PMID: 1d9be0209f0246b68da5a479a432aaf9.
Article16. Hu SQ, Hu JL, Zou FL, et al. 2022; P2X7 receptor in inflam-mation and pain. Brain Res Bull. 187:199–209. DOI: 10.1016/j.brainresbull.2022.07.006. PMID: 35850190.
Article17. Falk A, Koch P, Kesavan J, et al. 2012; Capture of neuroepithelial-like stem cells from pluripotent stem cells provides a versatile system for in vitro production of human neurons. PLoS One. 7:e29597. DOI: 10.1371/journal.pone.0029597. PMID: 22272239. PMCID: PMC3260177. PMID: 457e90b423754c62bf460dbcea8c338e.18. Yuan A, Sasaki T, Kumar A, et al. 2012; Peripherin is a subunit of peripheral nerve neurofilaments: implications for differential vulnerability of CNS and peripheral nervous system axons. J Neurosci. 32:8501–8508. DOI: 10.1523/JNEUROSCI.1081-12.2012. PMID: 22723690. PMCID: PMC3405552.
Article19. Sleigh JN, Dawes JM, West SJ, et al. 2017; Trk receptor signaling and sensory neuron fate are perturbed in human neuropathy caused by Gars mutations. Proc Natl Acad Sci U S A. 114:E3324–E3333. DOI: 10.1073/pnas.1614557114. PMID: 28351971. PMCID: PMC5402433.20. Mehta B, Goodman S, DiCarlo E, et al. 2023; Machine learning identification of thresholds to discriminate osteoarthritis and rheumatoid arthritis synovial inflammation. Arthritis Res Ther. 25:31. DOI: 10.1186/s13075-023-03008-8. PMID: 36864474. PMCID: PMC9979511. PMID: 7177784a35f941ac81d084f7ffcd9e30.
Article21. Han D, Fang Y, Tan X, et al. 2020; The emerging role of fibroblast-like synoviocytes-mediated synovitis in osteoarthritis: an update. J Cell Mol Med. 24:9518–9532. DOI: 10.1111/jcmm.15669. PMID: 32686306. PMCID: PMC7520283.
Article22. Ai M, Lin S, Zhang M, et al. 2021; Cirsilineol attenuates LPS-induced inflammation in both in vivo and in vitro models via inhibiting TLR-4/NFkB/IKK signaling pathway. J Bio-chem Mol Toxicol. 35:e22799. DOI: 10.1002/jbt.22799. PMID: 33949057.
Article23. Yan L, Liang M, Yang T, et al. 2020; The immunoregulatory role of myeloid-derived suppressor cells in the pathogenesis of rheumatoid arthritis. Front Immunol. 11:568362. DOI: 10.3389/fimmu.2020.568362. PMID: 33042149. PMCID: PMC7522347. PMID: 02c454b9e2a64c08bbdb2b1b66feed62.
Article24. Köles L, Leichsenring A, Rubini P, Illes P. 2011; P2 receptor signaling in neurons and glial cells of the central nervous sys-tem. Adv Pharmacol. 61:441–493. DOI: 10.1016/B978-0-12-385526-8.00014-X. PMID: 21586367.
Article25. Lin J, Sun AR, Li J, et al. 2021; A three-dimensional co-culture model for rheumatoid arthritis pannus tissue. Front Bioeng Biotechnol. 9:764212. DOI: 10.3389/fbioe.2021.764212. PMID: 34869276. PMCID: PMC8638776. PMID: a05aa82a38aa483ab7c6e7baae2a6070.
Article26. Rothbauer M, Höll G, Eilenberger C, et al. 2020; Monitoring tissue-level remodelling during inflammatory arthritis using a three-dimensional synovium-on-a-chip with non-invasive light scattering biosensing. Lab Chip. 20:1461–1471. DOI: 10.1039/C9LC01097A. PMID: 32219235.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Insulin enhances neurite extension and myelination of diabetic neuropathy neurons
- Mechanism of Neuronal Migration in Human Foetal Cerebrum In-vitro
- In Vitro Models Mimicking Immune Response in the Skin
- Evaluation of Medium Preparations for Temporomandibular Joint Disc Chondrocytes
- A Study on the Formation of Organotypic Spheroids from Early Human Fetal Brain