Int J Stem Cells.
2024 Feb;17(1):59-69. 10.15283/ijsc23037.
Expression of Major Histocompatibility Complex during Neuronal Differentiation of Somatic Cell Nuclear Transfer-Human Embryonic Stem Cells
- Affiliations
-
- 1Graduate School of Biomedical Science and Engineering, Hanyang University, Seoul, Korea
- 2CHA Advanced Research Institute, CHA University, Seongnam, Korea
- 3Paeanbiotechnology, Seoul, Korea
- 4Division of Biotechnology, College of Life Sciences and Biotechnology, Korea University, Seoul, Korea
- 5Department of Premedicine, College of Medicine, Hanyang University, Seoul, Korea
- 6Hanyang Institute of Bioscience and Biotechnology, Hanyang University, Seoul, Korea
- 7Hanyang Biomedical Research Institute, Hanyang University, Seoul, Korea
- 8Department of Biomedical Science, College of Life Science, CHA University, Seongnam, Korea
- KMID: 2552018
- DOI: http://doi.org/10.15283/ijsc23037
Abstract
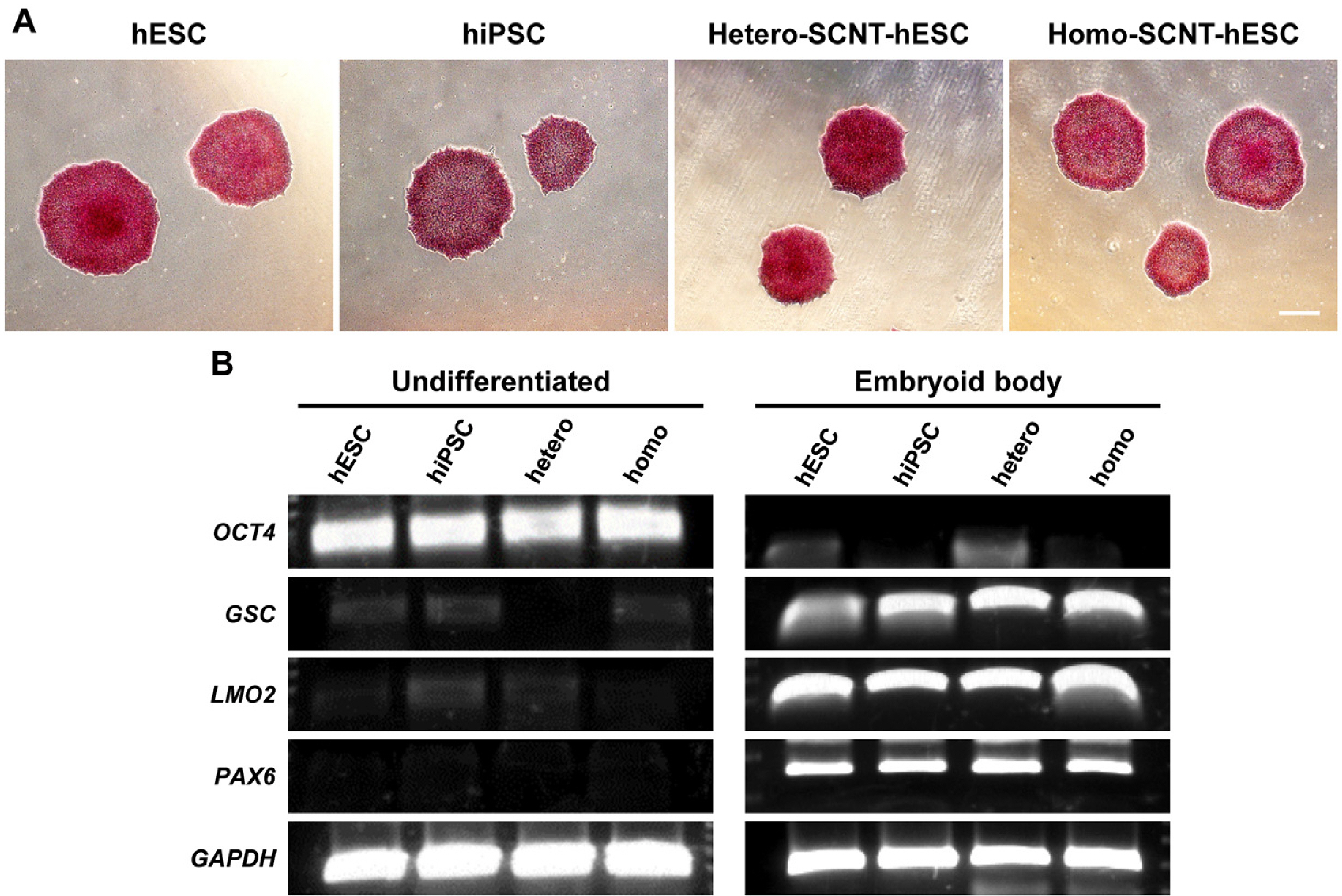
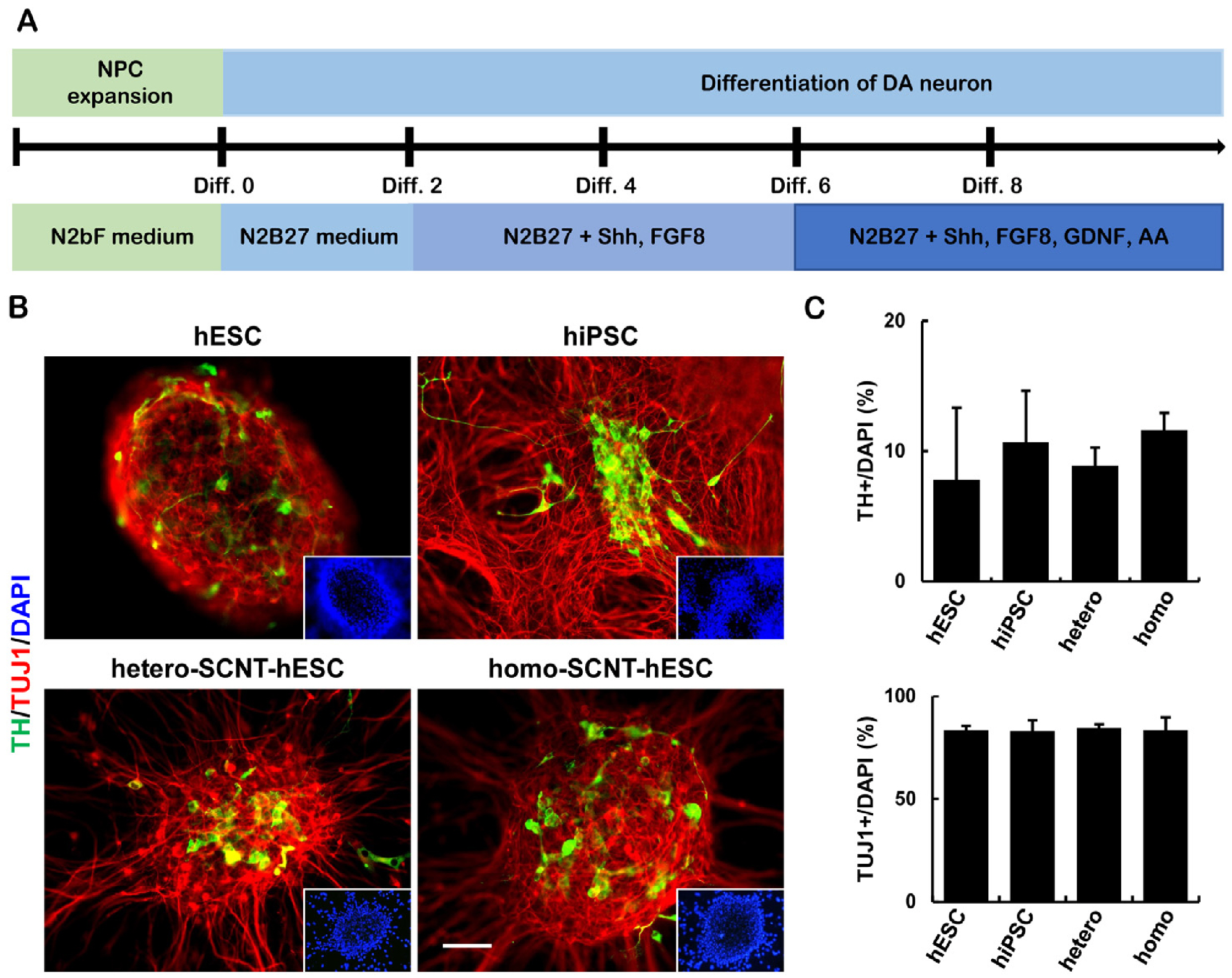
- Human pluripotent stem cells (hPSCs) such as human embryonic stem cells (hESCs), induced pluripotent stem cells, and somatic cell nuclear transfer (SCNT)-hESCs can permanently self-renew while maintaining their capacity to differentiate into any type of somatic cells, thereby serving as an important cell source for cell therapy. However, there are persistent challenges in the application of hPSCs in clinical trials, where one of the most significant is graft rejection by the patient immune system in response to human leukocyte antigen (HLA) mismatch when transplants are obtained from an allogeneic (non-self) cell source. Homozygous SCNT-hESCs (homo-SCNT-hESCs) were used to simplify the clinical application and to reduce HLA mismatch. Here, we present a xeno-free protocol that confirms the efficient generation of neural precursor cells in hPSCs and also the differentiation of dopaminergic neurons. Additionally, there was no difference when comparing the HLA expression patterns of hESC, homo-SCNT-hESCs and hetero-SCNT-hESCs. We propose that there are no differences in the differentiation capacity and HLA expression among hPSCs that can be cultured in vitro. Thus, it is expected that homo-SCNT-hESCs will possess a wider range of applications when transplanted with neural precursor cells in the context of clinical trials.
Figure
Reference
-
References
1. Cho MS, Hwang DY, Kim DW. 2008; Efficient derivation of functional dopaminergic neurons from human embryonic stem cells on a large scale. Nat Protoc. 3:1888–1894. DOI: 10.1038/nprot.2008.188. PMID: 19008875.
Article2. Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. 1998; Embryonic stem cell lines derived from human blastocysts. Science. 282:1145–1147. Erratum in: Science 1998;282:1827. DOI: 10.1126/science.282.5391.1145. PMID: 9804556.
Article3. Chung YG, Eum JH, Lee JE, et al. 2014; Human somatic cell nuclear transfer using adult cells. Cell Stem Cell. 14:777–780. DOI: 10.1016/j.stem.2014.03.015. PMID: 24746675.
Article4. Matoba S, Liu Y, Lu F, et al. 2014; Embryonic development following somatic cell nuclear transfer impeded by persisting histone methylation. Cell. 159:884–895. DOI: 10.1016/j.cell.2014.09.055. PMID: 25417163. PMCID: PMC4243038.
Article5. Chung YG, Matoba S, Liu Y, et al. 2015; Histone demethylase expression enhances human somatic cell nuclear transfer efficiency and promotes derivation of pluripotent stem cells. Cell Stem Cell. 17:758–766. DOI: 10.1016/j.stem.2015.10.001. PMID: 26526725.
Article6. Takahashi K, Tanabe K, Ohnuki M, et al. 2007; Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 131:861–872. DOI: 10.1016/j.cell.2007.11.019. PMID: 18035408.
Article7. Schwartz SD, Hubschman JP, Heilwell G, et al. 2012; Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 379:713–720. DOI: 10.1016/S0140-6736(12)60028-2. PMID: 22281388.
Article8. Kim JH, Auerbach JM, Rodríguez-Gómez JA, et al. 2002; Dopa-mine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature. 418:50–56. DOI: 10.1038/nature00900. PMID: 12077607.
Article9. Laflamme MA, Chen KY, Naumova AV, et al. 2007; Cardiomyo-cytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 25:1015–1024. DOI: 10.1038/nbt1327. PMID: 17721512.
Article10. van Berlo JH, Molkentin JD. 2014; An emerging consensus on cardiac regeneration. Nat Med. 20:1386–1393. DOI: 10.1038/nm.3764. PMID: 25473919. PMCID: PMC4418535.
Article11. Williams RC, Opelz G, Weil EJ, McGarvey CJ, Chakkera HA. 2017; The risk of transplant failure with HLA mismatch in first adult kidney allografts 2: living donors, summary, guide. Transplant Direct. 3:e152. DOI: 10.1097/TXD.0000000000000664. PMID: 28573187. PMCID: PMC5441983.
Article12. Gottwald W. 1978; Neurologic and psychiatric syndromes in lupus erythematosus. Z Hautkr. 53:505–514. German.13. Albert ML, Sauter B, Bhardwaj N. 1998; Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 392:86–89. DOI: 10.1038/32183. PMID: 9510252.
Article14. Doyle C, Strominger JL. 2010; Interaction between CD4 and class II MHC molecules mediates cell adhesion. 1987. J Immunol. 184:5935–5938. DOI: 10.1038/330256a0. PMID: 2823150.15. Ichise H, Nagano S, Maeda T, et al. 2017; NK cell alloreactivity against KIR-ligand-mismatched HLA-haploidentical tissue derived from HLA haplotype-homozygous iPSCs. Stem Cell Reports. 9:853–867. DOI: 10.1016/j.stemcr.2017.07.020. PMID: 28867344. PMCID: PMC5599245. PMID: 55716d85db5e4dffa93d13d9157f0cc5.
Article16. Kruse V, Hamann C, Monecke S, et al. 2015; Human induced pluripotent stem cells are targets for allogeneic and autologous natural killer (NK) cells and killing is partly mediated by the activating NK receptor DNAM-1. PLoS One. 10:e0125544. DOI: 10.1371/journal.pone.0125544. PMID: 25950680. PMCID: PMC4423859. PMID: ef081892998f45fa9fea252226fc72b1.
Article17. McGranahan N, Rosenthal R, Hiley CT, et al. 2017; Allele-specific HLA loss and immune escape in lung cancer evolution. Cell. 171:1259–1271.e11. DOI: 10.1016/j.cell.2017.10.001. PMID: 29107330. PMCID: PMC5720478.18. Lee KW, Oh DH, Lee C, Yang SY. 2005; Allelic and haplotypic diversity of HLA-A, -B, -C, -DRB1, and -DQB1 genes in the Korean population. Tissue Antigens. 65:437–447. DOI: 10.1111/j.1399-0039.2005.00386.x. PMID: 15853898.
Article19. Yang G, Deng YJ, Hu SN, et al. 2006; HLA-A, -B, and -DRB1 polymorphism defined by sequence-based typing of the Han population in Northern China. Tissue Antigens. 67:146–152. DOI: 10.1111/j.1399-0039.2006.00529.x. PMID: 16441486.
Article20. Lee EH, Park CH. 2017; Comparison of reprogramming methods for generation of induced-oligodendrocyte precursor cells. Biomol Ther (Seoul). 25:362–366. DOI: 10.4062/biomolther.2017.066. PMID: 28605832. PMCID: PMC5499613.
Article21. Mandai M, Watanabe A, Kurimoto Y, et al. 2017; Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med. 376:1038–1046. DOI: 10.1056/NEJMoa1608368. PMID: 28296613.
Article22. Lipsitz YY, Timmins NE, Zandstra PW. 2016; Quality cell therapy manufacturing by design. Nat Biotechnol. 34:393–400. DOI: 10.1038/nbt.3525. PMID: 27054995.
Article23. Chakradhar S. 2016; An eye to the future: researchers debate best path for stem cell-derived therapies. Nat Med. 22:116–119. DOI: 10.1038/nm0216-116. PMID: 26845400.
Article24. Krystkowiak P, Gaura V, Labalette M, et al. 2007; Alloimmunisa-tion to donor antigens and immune rejection following foetal neural grafts to the brain in patients with Huntington's disease. PLoS One. 2:e166. DOI: 10.1371/journal.pone.0000166. PMID: 17245442. PMCID: PMC1764859. PMID: a940106c9c5c4ba7a428d87fe610e8b6.
Article25. Brundin P, Pogarell O, Hagell P, et al. 2000; Bilateral caudate and putamen grafts of embryonic mesencephalic tissue treated with lazaroids in Parkinson's disease. Brain. 123(Pt 7):1380–1390. DOI: 10.1093/brain/123.7.1380. PMID: 10869050.
Article26. Barker RA, Drouin-Ouellet J, Parmar M. 2015; Cell-based therapies for Parkinson disease-past insights and future poten-tial. Nat Rev Neurol. 11:492–503. DOI: 10.1038/nrneurol.2015.123. PMID: 26240036.
Article27. Sette A, Sidney J. 1998; HLA supertypes and supermotifs: a functional perspective on HLA polymorphism. Curr Opin Im-munol. 10:478–482. DOI: 10.1016/S0952-7915(98)80124-6. PMID: 9722926.
Article28. Williams TM. 2001; Human leukocyte antigen gene polymor-phism and the histocompatibility laboratory. J Mol Diagn. 3:98–104. DOI: 10.1016/S1525-1578(10)60658-7. PMID: 11486048.
Article29. Gallardo D, Brunet S, Torres A, et al. 2004; Hla-DPB1 mismatch in HLA-A-B-DRB1 identical sibling donor stem cell transplantation and acute graft-versus-host disease. Transplantation. 77:1107–1110. DOI: 10.1097/01.TP.0000122225.10296.10. PMID: 15087781.
Article30. Park MH, Kim HS, Kang SJ. 1999; HLA-A,-B,-DRB1 allele and haplotype frequencies in 510 Koreans. Tissue Antigens. 53(4 Pt 1):386–390. DOI: 10.1034/j.1399-0039.1999.530412.x. PMID: 10323346.
Article31. Deuse T, Hu X, Gravina A, et al. 2019; Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat Biotechnol. 37:252–258. Erratum in: Nat Biotechnol 2022;40:1690. DOI: 10.1038/s41587-022-01426-8. PMID: 36114291. PMCID: PMC6419516.
Article32. Xu H, Wang B, Ono M, et al. 2019; Targeted disruption of HLA genes via CRISPR-Cas9 generates iPSCs with enhanced immune compatibility. Cell Stem Cell. 24:566–578.e7. DOI: 10.1016/j.stem.2019.02.005. PMID: 30853558.
Article33. Morizane A, Kikuchi T, Hayashi T, et al. 2017; MHC matching improves engraftment of iPSC-derived neurons in non-human primates. Nat Commun. 8:385. DOI: 10.1038/s41467-017-00926-5. PMID: 28855509. PMCID: PMC5577234. PMID: 37518cb36fd0402692f5bdd69bd784da.
Article34. Araki R, Uda M, Hoki Y, et al. 2013; Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature. 494:100–104. DOI: 10.1038/nature11807. PMID: 23302801.
Article35. Morizane A, Doi D, Kikuchi T, et al. 2013; Direct comparison of autologous and allogeneic transplantation of iPSC-derived neural cells in the brain of a non-human primate. Stem Cell Reports. 1:283–292. DOI: 10.1016/j.stemcr.2013.08.007. PMID: 24319664. PMCID: PMC3849265.
Article36. Zhao T, Zhang ZN, Rong Z, Xu Y. 2011; Immunogenicity of induced pluripotent stem cells. Nature. 474:212–215. DOI: 10.1038/nature10135. PMID: 21572395.
Article37. de Almeida PE, Meyer EH, Kooreman NG, et al. 2014; Trans-planted terminally differentiated induced pluripotent stem cells are accepted by immune mechanisms similar to self-tolerance. Nat Commun. 5:3903. DOI: 10.1038/ncomms4903. PMID: 24875164. PMCID: PMC4075468.
Article38. Zhao T, Zhang ZN, Westenskow PD, et al. 2015; Humanized mice reveal differential immunogenicity of cells derived from autologous induced pluripotent stem cells. Cell Stem Cell. 17:353–359. DOI: 10.1016/j.stem.2015.07.021. PMID: 26299572. PMCID: PMC9721102.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Human Embryonic Stem Cell Derived from Early Stage Fertilized Ovum: Non Immunogenic and Universal, Neuronal and Non-neuronal Cell Lines
- Current Concepts of Stem Cell Therapy
- Human embryonic stem cells and therapeutic cloning
- Ethical Issues on Embryonic Stem Cell Research
- Nuclear receptor regulation of stemness and stem cell differentiation