Ann Lab Med.
2023 Sep;43(5):451-460. 10.3343/alm.2023.43.5.451.
Effect of Lymphocyte Phenotypic Alterations on the Humoral Response to Vaccination Against SARS-COV-2 in Dialysis Patients
- Affiliations
-
- 1Department of Nephrology, Aristotle University of Thessaloniki, Hippokration Hospital, Thessaloniki, Greece
- 2Department of Immunology, National Peripheral Histocompatibility Center, Hippokration Hospital, Thessaloniki, Greece
- KMID: 2551993
- DOI: http://doi.org/10.3343/alm.2023.43.5.451
Abstract
- Background
The response to vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) varies depending on comorbidities. This study evaluated the clinical and immunological factors affecting the humoral response of patients with end-stage renal disease (ESRD) to the BNT162b2 vaccine.
Methods
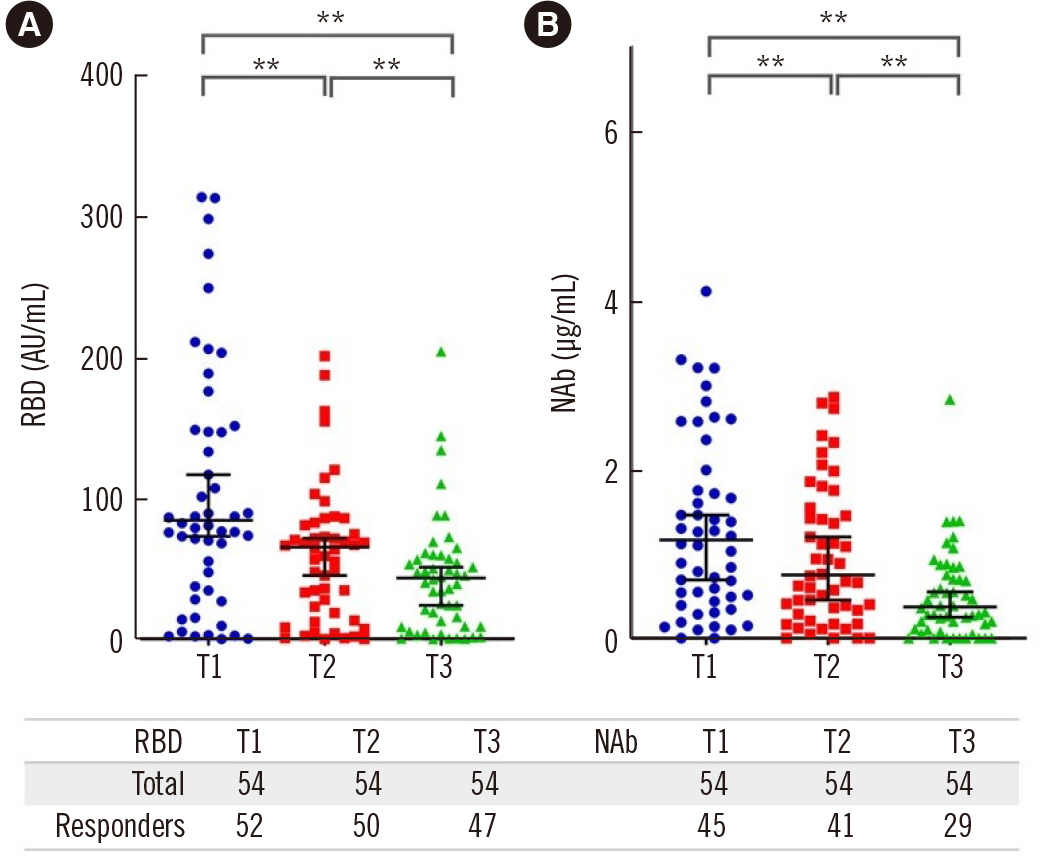
Humoral immunity was evaluated in 54 ESRD patients using serum levels of anti-receptor-binding domain (RBD) and neutralizing antibodies (NAbs), measured by a chemiluminescent immunoassay 30 (T1), 60 (T2), and 120 (T3) days after the second vaccine dose. The results were correlated to baseline patient T- and B-lymphocyte subpopulations determined by flow cytometry.
Results
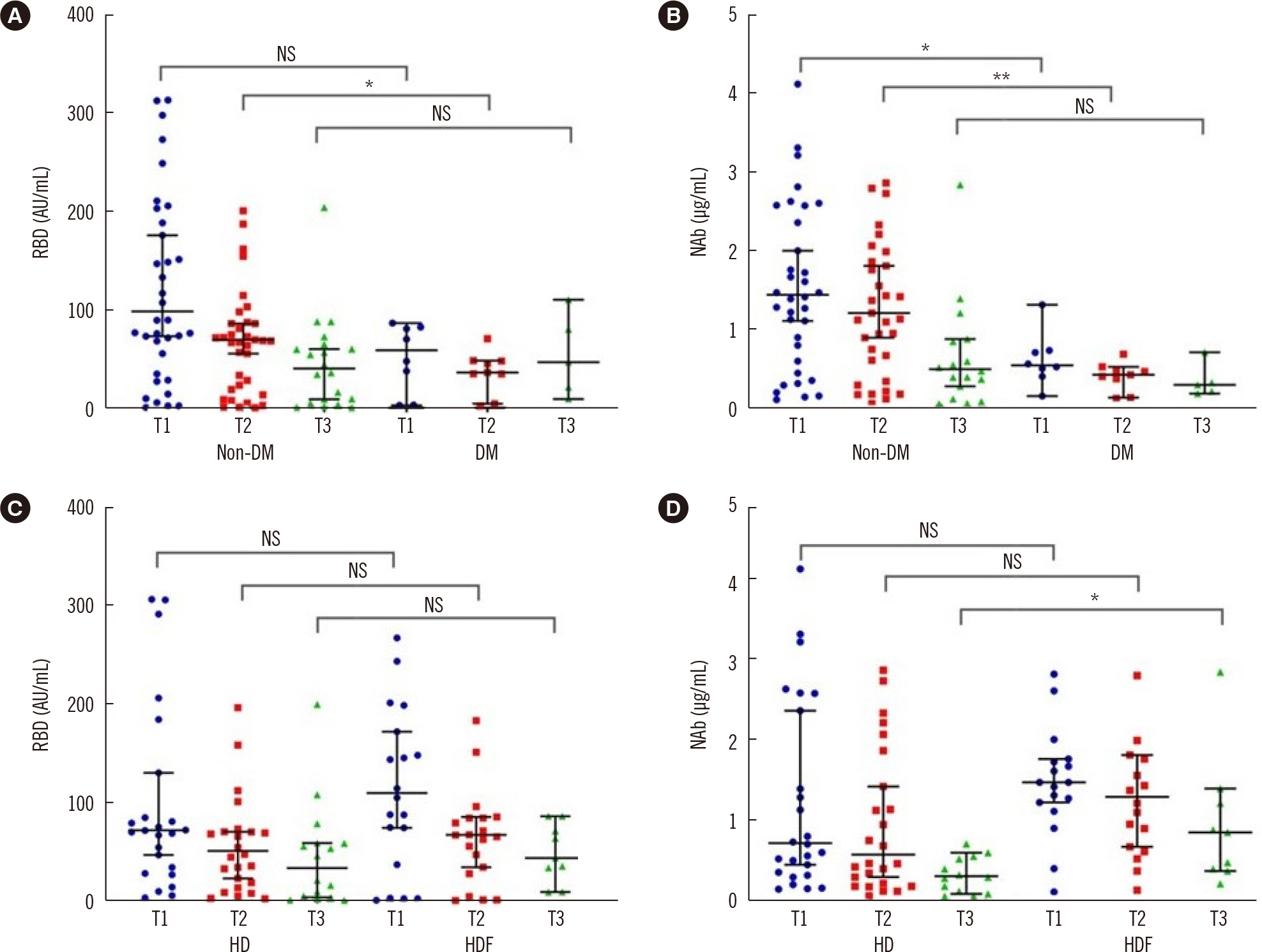
The proportion of seroconverted patients based on the NAb titer decreased from 83.3% at Τ1 to 53.7% at Τ3. Age was negatively correlated to the NAb titer at T1 and Τ2. Patients receiving hemodiafiltration had higher NAb titers at T3. Diabetes was associated with a lower response rate at T3. Univariate analysis revealed a positive correlation between the naïve CD4 T-lymphocyte population and RBD titer at T1 and the NAb titer at T3, with no association observed with naïve CD8 T lymphocytes. NAb titers at T3 were significantly correlated with late-differentiated CD4 T lymphocytes and terminally differentiated effector memory cells re-expressing CD45RA (TEMRA) CD8 T lymphocytes. RBD levels were positively correlated with naïve and memory B-lymphocyte counts at T3.
Conclusions
Age, diabetes, and hemodialysis prescription had significant impacts on the response to vaccination. T- and B-lymphocyte phenotypes are major determinants of the humoral response potency to SARS-CoV-2 vaccination with BNT162b2 in patients with ESRD.
Keyword
Figure
Cited by 1 articles
-
New Insights Into SARS-CoV-2-specific Antibody Levels in Kidney Transplantation Recipients After Three Vaccination Doses
Hyunhye Kang, Eun-Jee Oh
Ann Lab Med. 2024;44(1):3-5. doi: 10.3343/alm.2024.44.1.3.
Reference
-
1. Islam N, Jdanov DA, Shkolnikov VM, Khunti K, Kawachi I, White M, et al. Effects of covid-19 pandemic on life expectancy and premature mortality in 2020: time series analysis in 37 countries. BMJ. 2021; 375:e066768. DOI: 10.1136/bmj-2021-066768. PMID: 34732390. PMCID: PMC8564739.
Article2. Zsichla L, Müller V. 2023; Risk factors of severe COVID-19: a review of host, viral and environmental factors. Viruses. 15:175. DOI: 10.3390/v15010175. PMID: 36680215. PMCID: PMC9863423.
Article3. Mittal A, Manjunath K, Ranjan RK, Kaushik S, Kumar S, Verma V. 2020; COVID-19 pandemic: insights into structure, function, and hACE2 receptor recognition by SARS-CoV-2. PLoS Pathog. 16:e1008762. DOI: 10.1371/journal.ppat.1008762. PMID: 32822426. PMCID: PMC7444525.
Article4. Lioulios G, Fylaktou A, Papagianni A, Stangou M. 2021; T cell markers recount the course of immunosenescence in healthy individuals and chronic kidney disease. Clin Immunol. 225:108685. DOI: 10.1016/j.clim.2021.108685. PMID: 33549833.
Article5. Cancro MP, Tomayko MM. 2021; Memory B cells and plasma cells: the differentiative continuum of humoral immunity. Immunol Rev. 303:72–82. DOI: 10.1111/imr.13016. PMID: 34396546.
Article6. Grupper A, Sharon N, Finn T, Cohen R, Israel M, Agbaria A, et al. 2021; Humoral response to the Pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis. Clin J Am Soc Nephrol. 16:1037–42. DOI: 10.2215/CJN.03500321. PMID: 33824157. PMCID: PMC8425628.
Article7. Chee YJ, Tan SK, Yeoh E. 2020; Dissecting the interaction between COVID-19 and diabetes mellitus. J Diabetes Investig. 11:1104–14. DOI: 10.1111/jdi.13326. PMID: 32558211. PMCID: PMC7323255.
Article8. Yan Z, Yang M, Lai CL. 2021; COVID-19 vaccinations: a comprehensive review of their safety and efficacy in special populations. Vaccines. 9:1097. DOI: 10.3390/vaccines9101097. PMID: 34696205. PMCID: PMC8539110.
Article9. den Hoedt CH, Bots ML, Grooteman MP, van der Weerd NC, Mazairac AH, Penne EL, et al. 2014; Online hemodiafiltration reduces systemic inflammation compared to low-flux hemodialysis. Kidney Int. 86:423–32. DOI: 10.1038/ki.2014.9. PMID: 24552852.
Article10. Lioulios G, Fylaktou A, Xochelli A, Sampani E, Tsouchnikas I, Giamalis P, et al. 2022; Clustering of end stage renal disease patients by dimensionality reduction algorithms according to lymphocyte senescence markers. Front Immunol. 13:841031. DOI: 10.3389/fimmu.2022.841031. PMID: 35615367. PMCID: PMC9126282.
Article11. Vaziri ND. 2014; Gut microbial translocation in the pathogenesis of systemic inflammation in patients with end-stage renal disease. Dig Dis Sci. 59:2020–2. DOI: 10.1007/s10620-014-3287-z. PMID: 25038737.
Article12. Cicin-Sain L, Smyk-Pearson S, Currier N, Byrd L, Koudelka C, Robinson T, et al. 2010; Loss of naive T cells and repertoire constriction predict poor response to vaccination in old primates. J Immunol. 184:6739–45. DOI: 10.4049/jimmunol.0904193. PMID: 20483749. PMCID: PMC3504654.
Article13. Freitas GRR, da Luz Fernandes M, Agena F, Jaluul O, Silva SC, Lemos FBC, et al. 2019; Aging and end stage renal disease cause a decrease in absolute circulating lymphocyte counts with a shift to a memory profile and diverge in Treg population. Aging Dis. 10:49–61. DOI: 10.14336/AD.2018.0318. PMID: 30705767. PMCID: PMC6345336.
Article14. Wagner A, Garner-Spitzer E, Jasinska J, Kollaritsch H, Stiasny K, Kundi M, et al. 2018; Age-related differences in humoral and cellular immune responses after primary immunisation: indications for stratified vaccination schedules. Sci Rep. 8:9825. DOI: 10.1038/s41598-018-28111-8. PMID: 29959387. PMCID: PMC6026142.
Article15. Sampani E, Daikidou DV, Lioulios G, Xochelli A, Mitsoglou Z, Nikolaidou V, et al. 2021; CD28null and regulatory T cells are substantially disrupted in patients with end-stage renal disease due to diabetes mellitus. Int J Mol Sci. 22:2975. DOI: 10.3390/ijms22062975. PMID: 33804135. PMCID: PMC8001943.
Article16. Bergström M, Joly AL, Seiron P, Isringhausen S, Modig E, Fellström B, et al. 2015; Immunological profiling of haemodialysis patients and young healthy individuals with implications for clinical regulatory T cell sorting. Scand J Immunol. 81:318–24. DOI: 10.1111/sji.12287. PMID: 25737071.
Article17. Powell JD, Ragheb JA, Kitagawa-Sakakida S, Schwartz RH. 1998; Molecular regulation of interleukin-2 expression by CD28 co-stimulation and anergy. Immunol Rev. 165:287–300. DOI: 10.1111/j.1600-065X.1998.tb01246.x. PMID: 9850868.
Article18. Callender LA, Carroll EC, Beal RWJ, Chambers ES, Nourshargh S, Akbar AN, et al. 2018; Human CD8+ EMRA T cells display a senescence-associated secretory phenotype regulated by p38 MAPK. Aging Cell. 17:e12675. DOI: 10.1111/acel.12675. PMID: 29024417. PMCID: PMC5770853.19. Spolski R, Leonard WJ. 2014; Interleukin-21: a double-edged sword with therapeutic potential. Nat Rev Drug Discov. 13:379–95. DOI: 10.1038/nrd4296. PMID: 24751819.
Article20. Zamauskaite A, Perez-Cruz I, Yaqoob MM, Madrigal JA, Cohen SB. 1999; Effect of renal dialysis therapy modality on T cell cytokine production. Nephrol Dial Transplant. 14:49–55. DOI: 10.1093/ndt/14.1.49. PMID: 10052476.
Article21. Libetta C, Rampino T, Dal Canton A. 2001; Polarization of T-helper lymphocytes toward the Th2 phenotype in uremic patients. Am J Kidney Dis. 38:286–95. DOI: 10.1053/ajkd.2001.26092. PMID: 11479154.
Article22. Mansouri L, Nopp A, Jacobson SH, Hylander B, Lundahl J. 2017; Hemodialysis patients display a declined proportion of Th2 and regulatory T cells in parallel with a high interferon-γ profile. Nephron. 136:254–60. DOI: 10.1159/000471814. PMID: 28380480.
Article23. Zold E, Szodoray P, Kappelmayer J, Gaal J, Csathy L, Barath S, et al. 2010; Impaired regulatory T-cell homeostasis due to vitamin D deficiency in undifferentiated connective tissue disease. Scand J Rheumatol. 39:490–7. DOI: 10.3109/03009741003781951. PMID: 20615161.
Article24. Charoenngam N, Holick MF. 2020; Immunologic effects of vitamin D on human health and disease. Nutrients. 12:2097. DOI: 10.3390/nu12072097. PMID: 32679784. PMCID: PMC7400911.
Article25. Agüera ML, Hurtarte AR, Álvarez de Lara MA, Aumente MD, Carmona A, Carracedo J, et al. 2016; Donor-specific antibodies after starting hemodialysis in nonrenal solid organ transplant recipients: role of TH17. Transplant Proc. 48:2920–3. DOI: 10.1016/j.transproceed.2016.10.003. PMID: 27932108.
Article26. Lang CL, Wang MH, Hung KY, Hsu SH, Chiang CK, Lu KC. 2014; Correlation of interleukin-17-producing effector memory T cells and CD4+CD25+ Foxp3 regulatory T cells with the phosphate levels in chronic hemodialysis patients. ScientificWorldJournal. 2014:593170. DOI: 10.1155/2014/593170. PMID: 24558316. PMCID: PMC3914580.27. De Biasi S, Meschiari M, Gibellini L, Bellinazzi C, Borella R, Fidanza L, et al. 2020; Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat Commun. 11:3434. DOI: 10.1038/s41467-020-17292-4. PMID: 32632085. PMCID: PMC7338513.
Article28. Gil-Etayo FJ, Garcinuño S, Utrero-Rico A, Cabrera-Marante O, Arroyo-Sanchez D, Mancebo E, et al. 2022; An early Th1 response is a key factor for a favorable COVID-19 evolution. Biomedicines. 10:296. DOI: 10.3390/biomedicines10020296. PMID: 35203509. PMCID: PMC8869678.
Article29. Painter MM, Mathew D, Goel RR, Apostolidis SA, Pattekar A, Kuthuru O, et al. 2021; Rapid induction of antigen-specific CD4+ T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination. Immunity. 54:2133–42.e3. DOI: 10.1016/j.immuni.2021.08.001. PMID: 34453880. PMCID: PMC8361141.
Article30. Lim HW, Hillsamer P, Banham AH, Kim CH. 2005; Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J Immunol. 175:4180–3. DOI: 10.4049/jimmunol.175.7.4180. PMID: 16177055.31. Garner-Spitzer E, Wagner A, Paulke-Korinek M, Kollaritsch H, Heinz FX, Redlberger-Fritz M, et al. 2013; Tick-borne encephalitis (TBE) and hepatitis B nonresponders feature different immunologic mechanisms in response to TBE and influenza vaccination with involvement of regulatory T and B cells and IL-10. J Immunol. 191:2426–36. DOI: 10.4049/jimmunol.1300293. PMID: 23872054.
Article32. Mathew RO, Mason DL, Song R, Tryniszewski T, Kennedy JS. 2016; Role of T-regulatory cells in the response to hepatitis B vaccine in hemodialysis patients. Hemodial Int. 20:242–52. DOI: 10.1111/hdi.12326. PMID: 26104830.
Article33. Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O'Fallon WM, Weyand CM. 2001; Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol. 75:12182–7. DOI: 10.1128/JVI.75.24.12182-12187.2001. PMID: 11711609. PMCID: PMC116115.
Article34. Saurwein-Teissl M, Lung TL, Marx F, Gschösser C, Asch E, Blasko I, et al. 2002; Lack of antibody production following immunization in old age: association with CD8(+)CD28(-) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 168:5893–9. DOI: 10.4049/jimmunol.168.11.5893. PMID: 12023394.
Article35. Alonso Arias R, Moro-García MA, Echeverría A, Solano-Jaurrieta JJ, Suárez-García FM, López-Larrea C. 2013; Intensity of the humoral response to cytomegalovirus is associated with the phenotypic and functional status of the immune system. J Virol. 87:4486–95. DOI: 10.1128/JVI.02425-12. PMID: 23388717. PMCID: PMC3624366.
Article36. Derhovanessian E, Theeten H, Hähnel K, Van Damme P, Cools N, Pawelec G. 2013; Cytomegalovirus-associated accumulation of late-differentiated CD4 T-cells correlates with poor humoral response to influenza vaccination. Vaccine. 31:685–90. DOI: 10.1016/j.vaccine.2012.11.041. PMID: 23196209.
Article37. Künzel W, Glathe H, Engelmann H, Van Hoecke C. 1996; Kinetics of humoral antibody response to trivalent inactivated split influenza vaccine in subjects previously vaccinated or vaccinated for the first time. Vaccine. 14:1108–10. DOI: 10.1016/0264-410X(96)00061-8. PMID: 8911005.
Article38. Pyhälä R, Kumpulainen V, Alanko S, Forsten T. 1994; HI antibody kinetics in adult volunteers immunized repeatedly with inactivated trivalent influenza vaccine in 1990-1992. Vaccine. 12:947–52. DOI: 10.1016/0264-410X(94)90039-6. PMID: 7975836.
Article39. Siegrist CA, Aspinall R. 2009; B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 9:185–94. DOI: 10.1038/nri2508. PMID: 19240757.
Article40. Gustafson CE, Kim C, Weyand CM, Goronzy JJ. 2020; Influence of immune aging on vaccine responses. J Allergy Clin Immunol. 145:1309–21. DOI: 10.1016/j.jaci.2020.03.017. PMID: 32386655. PMCID: PMC7198995.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Discrimination between Benign and Malignant Post-SARS-CoV-2 Vaccination Lymphadenopathy is Feasible
- New Insights Into SARS-CoV-2-specific Antibody Levels in Kidney Transplantation Recipients After Three Vaccination Doses
- Guillain-Barre Syndrome Following SARS-CoV-2 Vaccination: A Case Report
- New-Onset Fulminant Type 1 Diabetes Following SARS-CoV-2 Protein Subunit Vaccine: A Case Report and Literature Review
- Humoral immune response to SARS-CoV-2 mRNA vaccines is associated with choice of vaccine and systemic adverse reactions