Ann Lab Med.
2023 Jul;43(4):389-391. 10.3343/alm.2023.43.4.389.
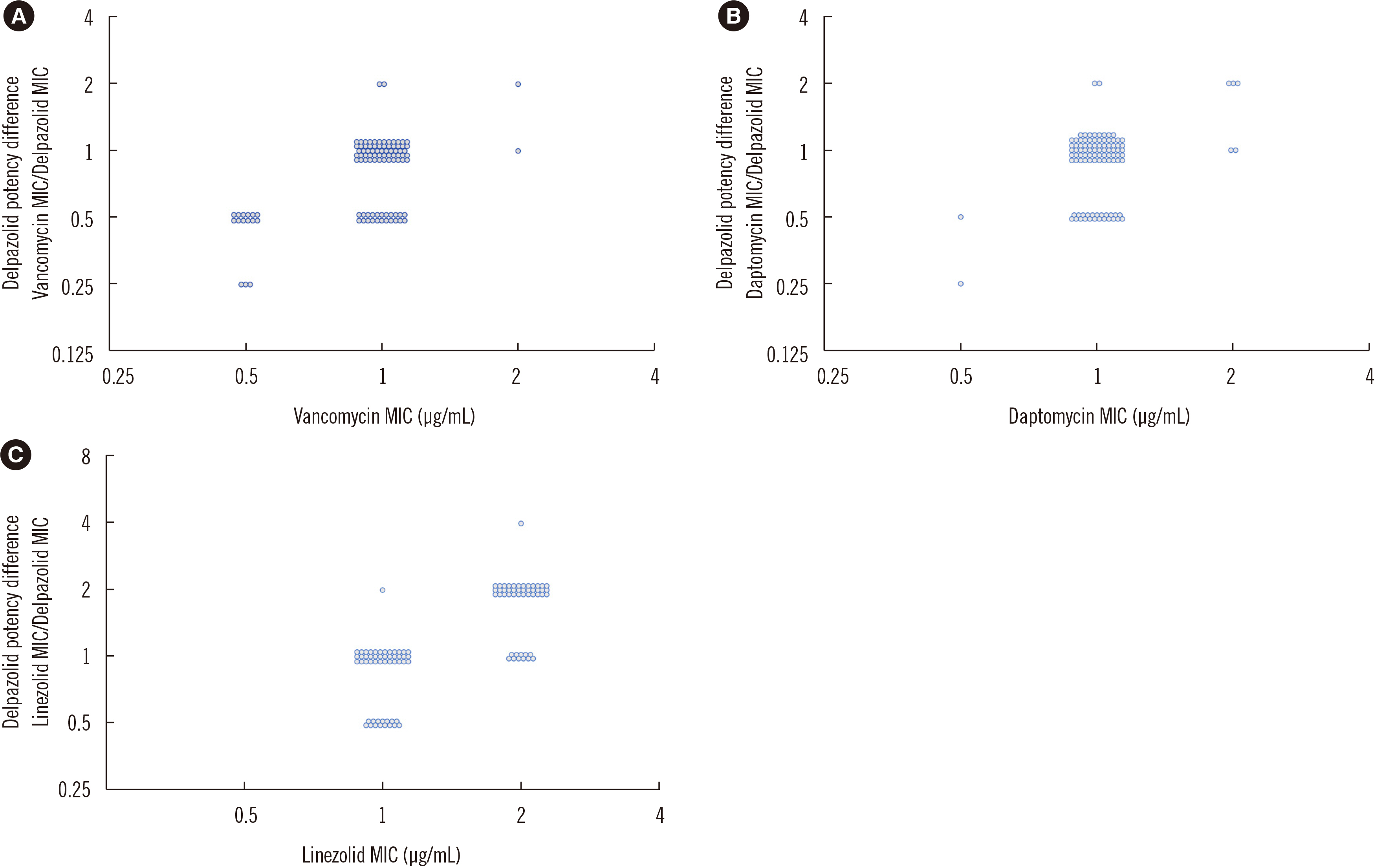
In-vitro Activity of Delpazolid and Comparator Agents Against Methicillin-resistant Staphylococcus aureus Involved in Bloodstream Infection
- Affiliations
-
- 1Department of Laboratory Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 2Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- KMID: 2551958
- DOI: http://doi.org/10.3343/alm.2023.43.4.389
Figure
Reference
-
1. Nannini E, Murray BE, Arias CA. 2010; Resistance or decreased susceptibility to glycopeptides, daptomycin, and linezolid in methicillin-resistant Staphylococcus aureus. Curr Opin Pharmacol. 10:516–21. DOI: 10.1016/j.coph.2010.06.006. PMID: 20598637.2. Narita M, Tsuji BT, Yu VL. 2007; Linezolid-associated peripheral and optic neuropathy, lactic acidosis, and serotonin syndrome. Pharmacotherapy. 27:1189–97. DOI: 10.1592/phco.27.8.1189. PMID: 17655517.
Article3. Lewis PO, Heil EL, Covert KL, Cluck DB. 2018; Treatment strategies for persistent methicillin-resistant Staphylococcus aureus bacteraemia. J Clin Pharm Ther. 43:614–25. DOI: 10.1111/jcpt.12743. PMID: 30003555.
Article4. Yu SH, Lee JH, Kim MC, Choi SH, Chung JW, Lee MK. 2021; Ten-year prevalence trends of phenotypically identified community-associated methicillin-resistant Staphylococcus aureus strains in clinical specimens. Ann Lab Med. 41:386–93. DOI: 10.3343/alm.2021.41.4.386. PMID: 33536357. PMCID: PMC7884191.
Article5. Cho YS, Lim HS, Cho YL, Nam HS, Bae KS. 2018; Multiple-dose safety, tolerability, pharmacokinetics, and pharmacodynamics of oral LCB01-0371 in healthy male volunteers. Clin Ther. 40:2050–64. DOI: 10.1016/j.clinthera.2018.10.007. PMID: 30420289.
Article6. Jeong JW, Jung SJ, Lee HH, Kim YZ, Park TK, Cho YL, et al. 2010; In vitro and in vivo activities of LCB01-0371, a new oxazolidinone. Antimicrob Agents Chemother. 54:5359–62. DOI: 10.1128/AAC.00723-10. PMID: 20855730. PMCID: PMC2981296.
Article7. Zong Z, Jing W, Shi J, Wen S, Zhang T, Huo F, et al. 2018; Comparison of in vitro activity and MIC distributions between the novel oxazolidinone delpazolid and linezolid against multidrug-resistant and extensively drug-resistant Mycobacterium tuberculosis in China. Antimicrob Agents Chemother. 62:e00165–18. DOI: 10.1128/AAC.00165-18. PMID: 29844043. PMCID: PMC6105784.8. CLSI. 2021. Performance standards for antimicrobial susceptibility testing, M100. 31th ed. Clinical and Laboratory Standards Institute;Wayne, PA:
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of multiple furunculosis caused by methicillin-resistant staphylococcs aureus
- Detection of Multidrug Resistant Patterns and Associated - genes of Methicillin Resistant Staphylococcus aureus ( MRSA ) Isolated from Clinical Specimens
- A statistical analysis of methicillin-resistant staphylococcus aureus
- Community-Associated Methicillin-Resistant Staphylococcus aureus in Nosocomial Infections
- Treatment of Methicillin-Resistant Staphylococcus aureus Bacteremia