Ann Lab Med.
2023 Mar;43(2):187-195. 10.3343/alm.2023.43.2.187.
Common Data Model-based Analysis of Selective Leukoreduction Protocol Compliance at Three Hospitals
- Affiliations
-
- 1Department of Laboratory Medicine and Genetics, Soonchunhyang University Bucheon Hospital, Soonchunhyang University College of Medicine, Bucheon, Korea
- 2Division of Nephrology, Department of Internal Medicine, Soonchunhyang University Bucheon Hospital, Soonchunhyang University College of Medicine, Bucheon, Korea
- 3Department of Laboratory Medicine, Soonchunhyang University Seoul Hospital, Soonchunhyang University College of Medicine, Seoul, Korea
- 4Department of Laboratory Medicine, Soonchunhyang University Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea
- KMID: 2551696
- DOI: http://doi.org/10.3343/alm.2023.43.2.187
Abstract
- Background
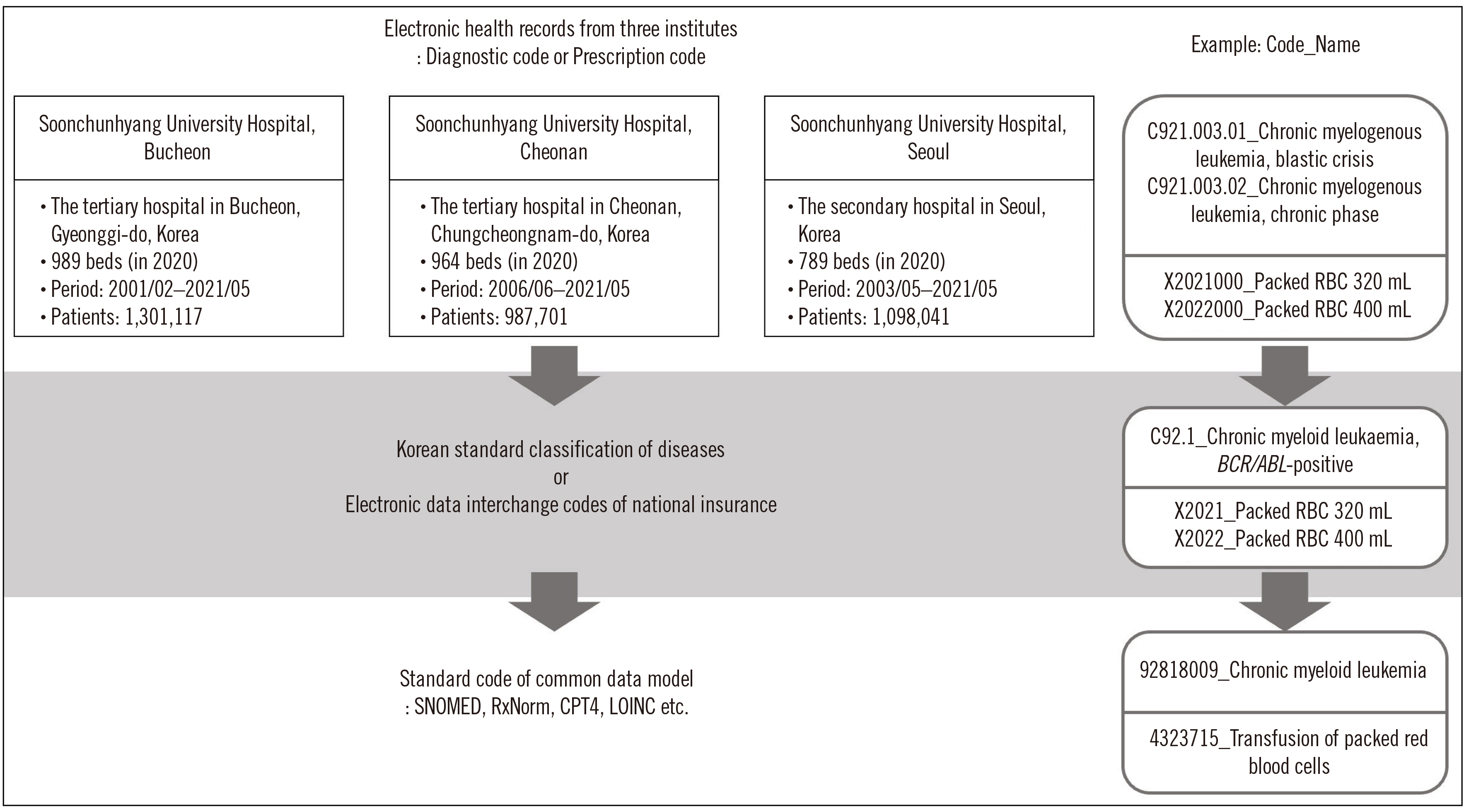
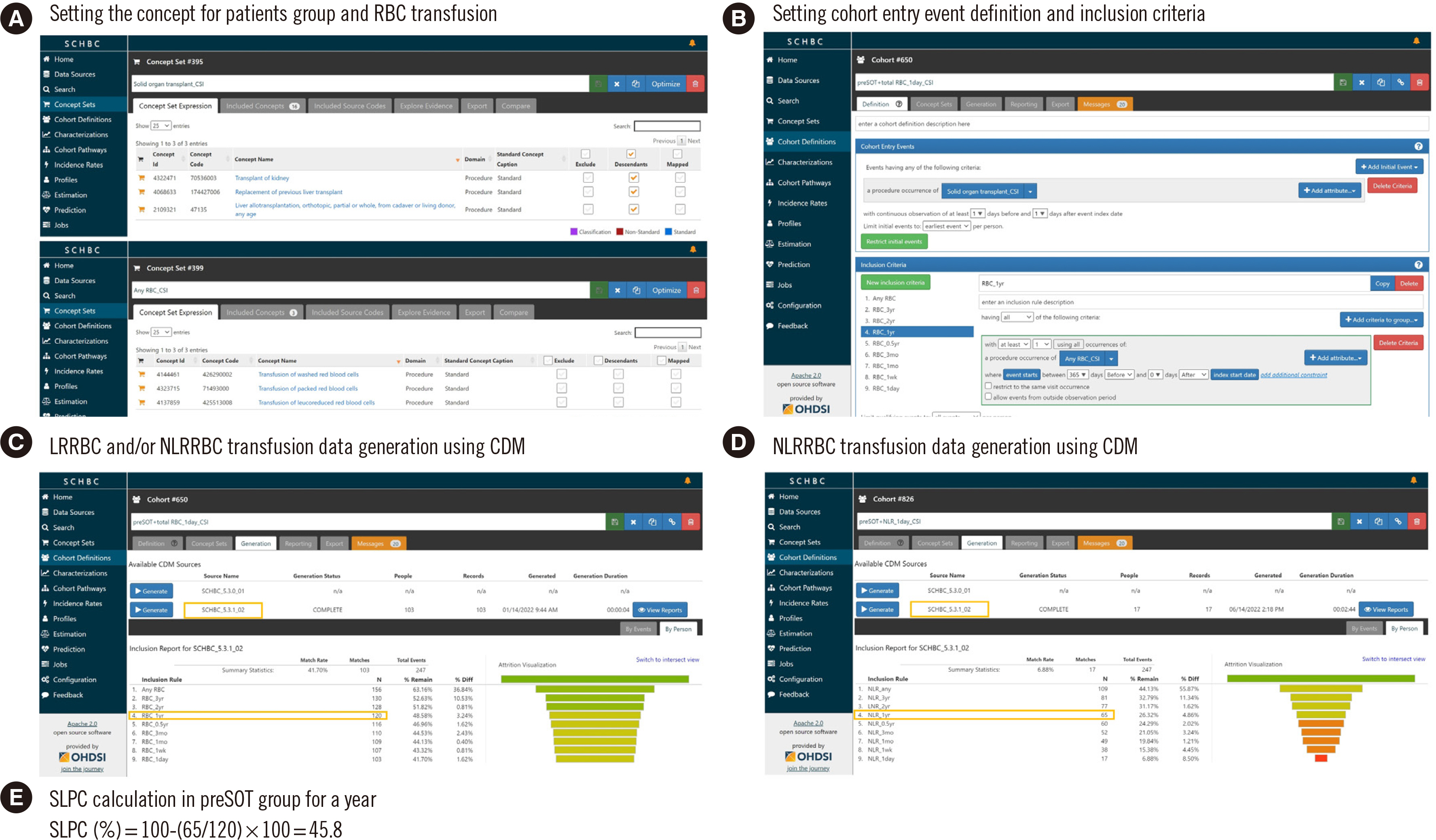
The selective leukoreduction protocol (SLP) is limited in that patients who require it can be overlooked. We estimated SLP compliance (SLPC) using the Observational Medical Outcomes Partnership common data model (CDM).
Methods
Patients were classified into eight groups: pre- and post-hematology disease (A and B), pre- and post-solid organ transplantation (C and D), solid cancer (E), immunodeficiency (F), anticancer therapy (G), and cardiovascular surgery (H). We examined the red blood cell (RBC) transfusion history from three hospital datasets comprising approximately three million patients over 20 years using CDM-based analysis. SLPC was calculated as the percentage of patients who received only leukoreduced RBCs in total patients transfused RBCs.
Results
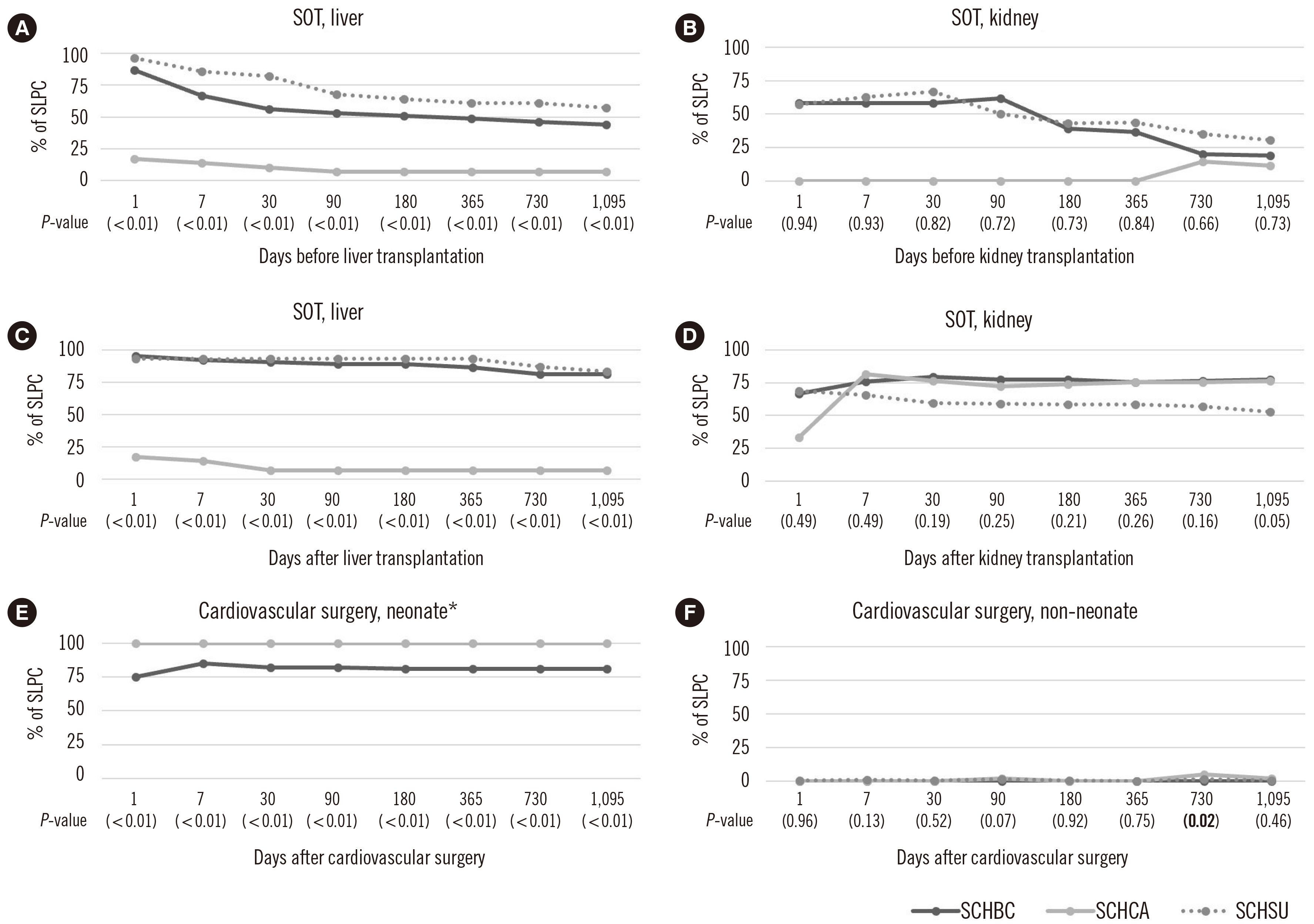
In total, 166,641 patients from three hospitals were enrolled in this study. From 2001 to 2021, SLPC in all groups, except H, tended to increase, although there were differences among the hospitals. Based on the most recent values (2017–2021), the SLPC in groups A, B, D, and G was maintained at ≥75% until 1,095 days before or after diagnosis or treatment. Groups E, F, and H had < 50% SLPC one day after diagnosis and treatment.
Conclusions
CDM analysis supports the review of large datasets for SLPC evaluation. Although SLPC tended to improve in most patient groups, additional education and monitoring are needed for groups that continue to show low SLPC.
Keyword
Figure
Reference
-
1. Ratko TA, Cummings JP, Oberman HA, Crookston KP, DeChristopher PJ, Eastlund DT, et al. 2001; Evidence‐based recommendations for the use of WBC‐reduced cellular blood components. Transfusion. 41:1310–9. DOI: 10.1046/j.1537-2995.2001.41101310.x. PMID: 11606834.
Article2. Bassuni WY, Blajchman MA, Al-Moshary MA. 2008; Why implement universal leukoreduction? Hematol. Oncol Stem Cell Ther. 1:106–23. DOI: 10.1016/S1658-3876(08)50042-2.
Article3. Choi SJ, Kim S, Kim HO, Kwon JR, Lee SW, Shin YH. 2012; The status of use of leukoreduced blood products in Korean hospitals. Lab Med Online. 2:204–8. DOI: 10.3343/lmo.2012.2.4.204.
Article4. Whitaker BI, Hinkins S. 2011; The 2011 national blood collection and utilization survey report. United States Department of Health and Human Services. 1–88.5. Trial to Reduce Alloimmunization to Platelets Study Group. 1997; Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. N Engl J Med. 337:1861–9. DOI: 10.1056/NEJM199712253372601. PMID: 9417523.6. Choi S, Choi SJ, Kim JK, Nam KC, Lee S, Kim JH, et al. 2021; Preliminary feasibility assessment of CDM-based active surveillance using current status of medical device data in medical records and OMOP-CDM. Sci Rep. 11:24070. DOI: 10.1038/s41598-021-03332-6. PMID: 34911976. PMCID: PMC8674329.
Article7. Hripcsak G, Duke JD, Shah NH, Reich CG, Huser V, Schuemie MJ, et al. 2015; Observational Health Data Sciences and Informatics (OHDSI): opportunities for observational researchers. Stud Health Technol Inform. 216:574–8.8. Park RW. 2019; The distributed research network, observational health data sciences and informatics, and the South Korean research network. Korean J Med. 94:309–14. DOI: 10.3904/kjm.2019.94.4.309.
Article9. Seong Y, You SC, Ostropolets A, Rho Y, Park J, Cho J, et al. 2021; Incorporation of Korean electronic data interchange vocabulary into observational medical outcomes partnership vocabulary. Healthc Inform Res. 27:29–38. DOI: 10.4258/hir.2021.27.1.29. PMID: 33611874. PMCID: PMC7921574.
Article10. Koo BN, Kwon MA, Kim SH, Kim JY, Moon YJ, Park SY, et al. 2019; Korean clinical practice guideline for perioperative red blood cell transfusion from Korean Society of Anesthesiologists. Korean J Anesthesiol. 72:91–118. DOI: 10.4097/kja.d.18.00322. PMID: 30513567. PMCID: PMC6458508.
Article11. National organ tissue blood management agency. 2022. Korean national transfusion guideline. 5th ed. National organ tissue blood management agency;Seoul: p. 56–8.12. Choi S, Hyun J, Yu H, Cho D. 2021; ABO-incompatible transfusion events reported in written judgments and in the Korean hemovigilance system. Ann Lab Med. 41:493–8. DOI: 10.3343/alm.2021.41.5.493. PMID: 33824239. PMCID: PMC8041592.
Article13. Sarkar S, Seshadri D. 2014; Conducting record review studies in clinical practice. J Clin Diagn Res. 8:JG01–04. DOI: 10.7860/JCDR/2014/8301.4806. PMID: 25386466. PMCID: PMC4225918.
Article14. Song MJ, Kim S, Boo D, Park C, Yoo S, Yoon HI, et al. 2021; Comparison of proton pump inhibitors and histamine 2 receptor antagonists for stress ulcer prophylaxis in the intensive care unit. Sci Rep. 11:18467. DOI: 10.1038/s41598-021-98069-7. PMID: 34531488. PMCID: PMC8446063.
Article15. Josephson CD, Glynn S, Mathew S, Birch R, Bakkour S, Baumann Kreuziger L, et al. 2022; The Recipient Epidemiology and Donor Evaluation Study‐IV‐Pediatric (REDS‐IV‐P): a research program striving to improve blood donor safety and optimize transfusion outcomes across the lifespan. Transfusion. 62:982–99. DOI: 10.1111/trf.16869. PMID: 35441384.
Article16. Leffell MS, Kim D, Vega RM, Zachary AA, Petersen J, Hart JM, et al. 2014; Red blood cell transfusions and the risk of allosensitization in patients awaiting primary kidney transplantation. Transplantation. 97:525–33. DOI: 10.1097/01.tp.0000437435.19980.8f. PMID: 24300013.
Article17. Choi S, Lee KW, Park JB, Kim K, Jang HR, Huh W, et al. 2020; C3d-positive preformed DSAs tend to persist and result in a higher risk of AMR after kidney transplants. J Clin Med. 9:375. DOI: 10.3390/jcm9020375. PMID: 32019081. PMCID: PMC7073748.
Article18. Wallis JP, Chapman CE, Orr KE, Clark SC, Forty JR. 2002; Effect of WBC reduction of transfused RBCs on postoperative infection rates in cardiac surgery. Transfusion. 42:1127–34. DOI: 10.1046/j.1537-2995.2002.00181.x. PMID: 12430668.
Article19. Gu YJ, devries AJ, Boonstra PW, van Oeveren W. 1995; Clinical performance of a high-efficiency rapid flow leucocyte removal filter for leucocyte depletion of heparinized cardiopulmonary bypass perfusate. Perfusion. 10:425–30. DOI: 10.1177/026765919501000606. PMID: 8747899.
Article20. Kim HI, Yoon JY, Kwak MS, Cha JM. 2021; Real-world use of colonoscopy in an older population: A nationwide standard cohort study using a common data model. Dig Dis Sci. 66:2227–34. DOI: 10.1007/s10620-020-06494-x. PMID: 32691386.
Article21. Yoon JY, Kwak MS, Kim HI, Cha JM. 2021; Seasonal variations in the diagnosis of the top 10 cancers in Korea: a nationwide population‐based study using a common data model. J Gastroenterol Hepatol. 36:3371–80. DOI: 10.1111/jgh.15634. PMID: 34293206.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development of a Structural Model Explaining Medication Compliance of Persons with Schizophrenia
- Is Leukoreduction Needed for Plasma Products?
- A Structural Analysis for Psychosocial Variables related to Sick Role Behavioral Compliance in Hemodialysis Patients
- The Status of Use of Leukoreduced Blood Products in Korean Hospitals
- Compliance with Recommendations on Work Schedule for Shift Nurses in South Korea