Ann Lab Med.
2023 Mar;43(2):153-166. 10.3343/alm.2023.43.2.153.
Comprehensive Evaluation of the NeoBase 2 Non-derivatized MSMS Assay and Exploration of Analytes With Significantly Different Concentrations Between Term and Preterm Neonates
- Affiliations
-
- 1Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2551692
- DOI: http://doi.org/10.3343/alm.2023.43.2.153
Abstract
- Background
Despite the popularity of the NeoBase 2 Non-derivatized MSMS assay (PerkinElmer, Turku, Finland), there are no reports of its comprehensive evaluation, including the ability to distinguish transient tyrosinemia of the newborn (TTN) from tyrosinemia type 1 (TYR 1) using succinylacetone (SUAC). No newborn screening (NBS) cutoffs for preterm neonates in the Korean population have been suggested. We evaluated the NeoBase 2 assay and identified analytes requiring different cutoffs in preterm neonates.
Methods
Residual NBS dried blood spot samples and proficiency testing (PT) materials of the Newborn Screening Quality Assurance Program and the Korean Association of External Quality Assessment Service were used. Precision, accuracy, limit of detection (LOD), lower limit of quantification (LLOQ), linearity, recovery, carryover, and performance of SUAC were evaluated. Cutoffs were determined, and analytes requiring different cutoffs in preterm neonates were investigated.
Results
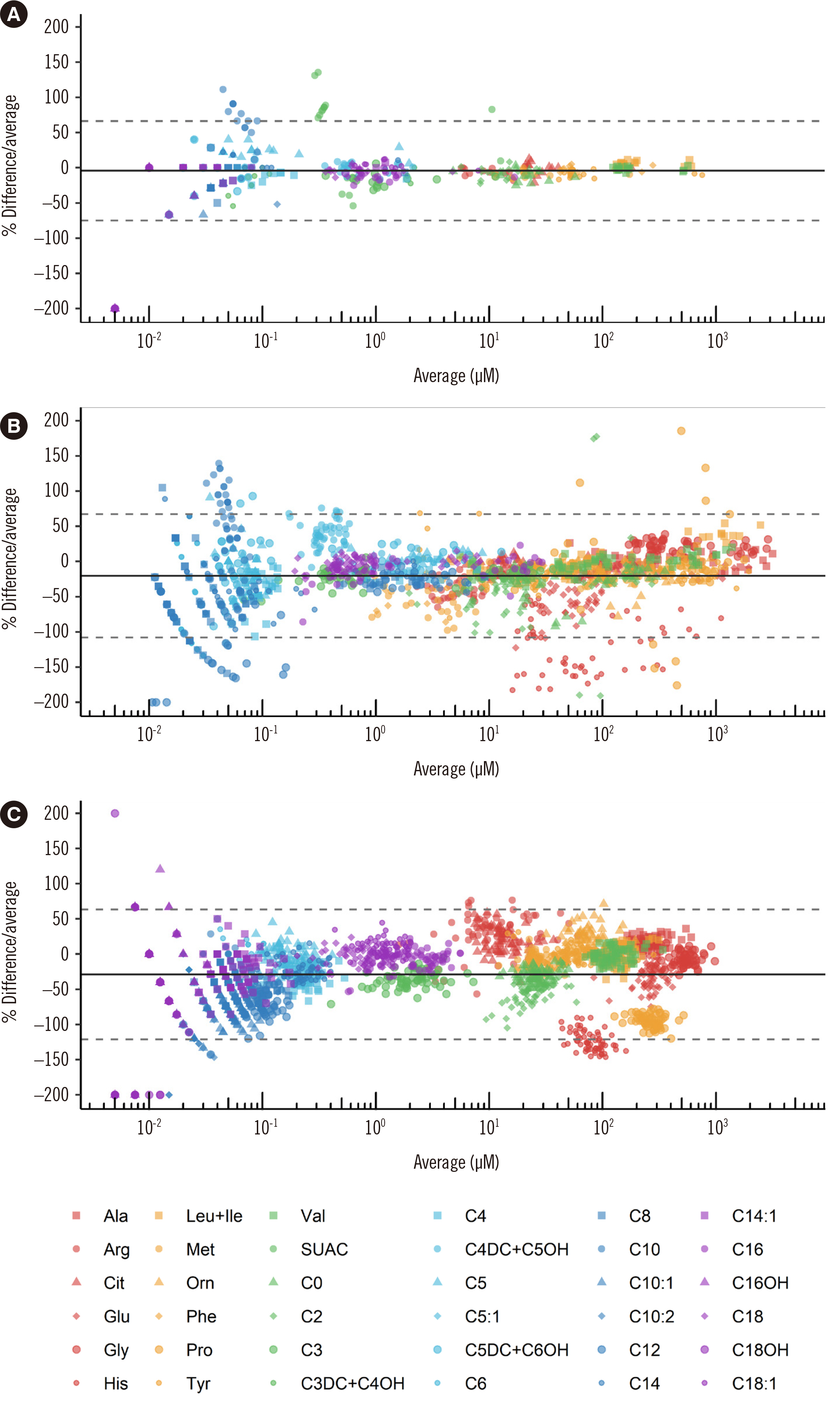
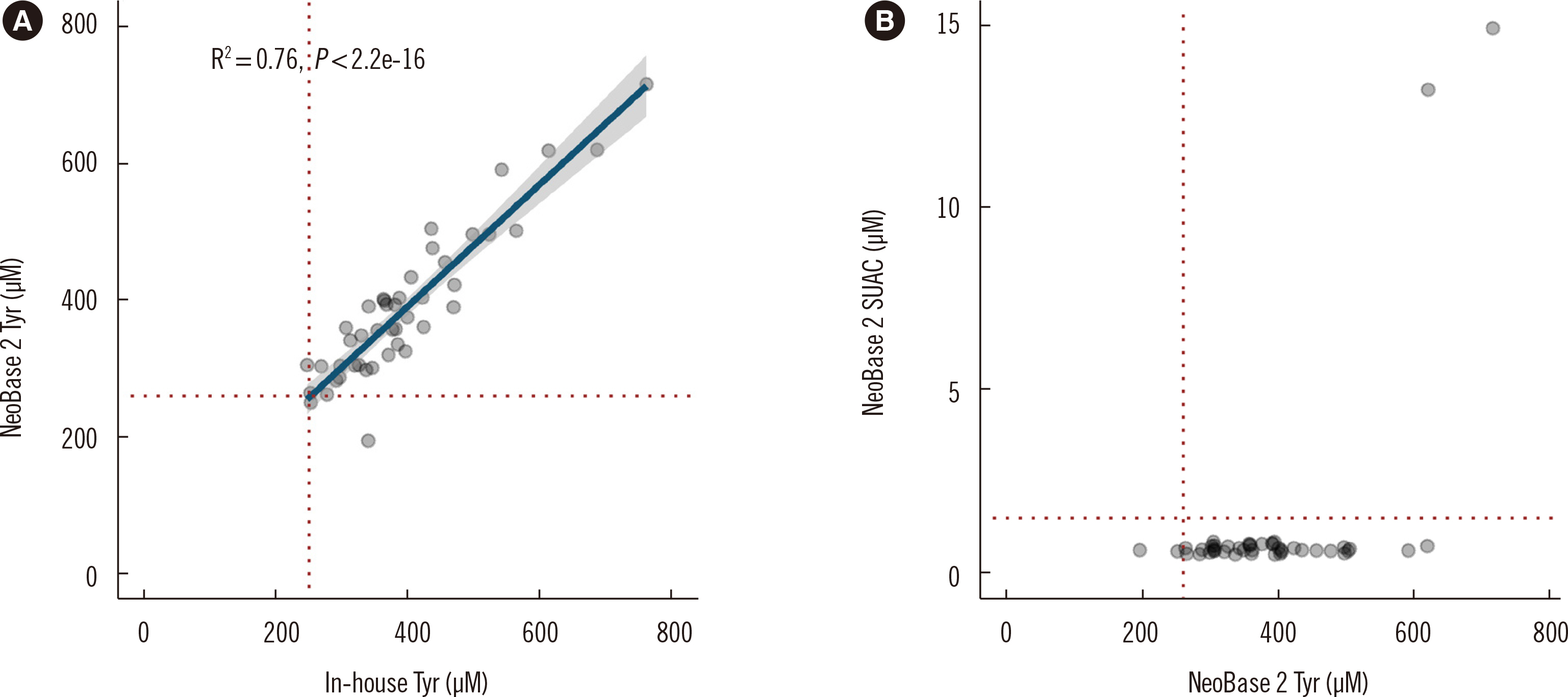
Mean CVs for within-run and between-day precision were within 15%. Accuracy analysis indicated high agreement with in-house derivatized assay results and results of other PT participants. All analytes demonstrated acceptable LOD, LLOQ, and linearity. Recoveries were acceptable, except for SUAC. Carryover was negligible. Cutoffs were established for all analytes; Tyr, adenosine, and C20:0-lysophosphatidylcholine required different cutoffs in preterm neonates. Differential diagnosis of TYR 1 and TTN was successful with simultaneous Tyr and SUAC measurement.
Conclusions
The NeoBase 2 assay demonstrated satisfactory performance. The additional analytes provide a wider diagnostic coverage, and the simultaneous measurement of Tyr and SUAC is efficient in excluding TYR 1. The new cutoffs for preterm neonates may decrease false-positive rates, without compromising diagnostic sensitivity.
Keyword
Figure
Cited by 1 articles
-
Non-derivatizing Tandem Mass Spectrometry Assay for Expanded Newborn Screening and Cutoffs for Preterm Neonates
Joon Hee Lee, Junghan Song
Ann Lab Med. 2023;43(2):133-134. doi: 10.3343/alm.2023.43.2.133.
Reference
-
1. Schulze A, Lindner M, Kohlmüller D, Olgemöller K, Mayatepek E, Hoffmann GF. 2003; Expanded newborn screening for inborn errors of metabolism by electrospray ionization-tandem mass spectrometry: results, outcome, and implications. Pediatrics. 111:1399–406. DOI: 10.1542/peds.111.6.1399. PMID: 12777559.
Article2. Wilcken B, Wiley V, Hammond J, Carpenter K. 2003; Screening newborns for inborn errors of metabolism by tandem mass spectrometry. N Engl J Med. 348:2304–12. DOI: 10.1056/NEJMoa025225. PMID: 12788994.
Article3. Centers for Disease Control and Prevention (CDC). Newborn Screening Quality Assurance Program (NSQAP) 2020 Quality control program report. https://www.cdc.gov/labstandards/pdf/nsqap/QC_Report_S1_2020-508.pdf. Updated on Jun 2020.4. Cicalini I, Valentinuzzi S, Pieragostino D, Consalvo A, Zucchelli M, Donzelli S, et al. 2021; Analytical evaluation of the ideal strategy for high-throughput flow injection analysis by tandem mass spectrometry in routine newborn screening. Metabolites. 11:473. DOI: 10.3390/metabo11080473. PMID: 34436414. PMCID: PMC8399422.
Article5. Zheng Y, Chen Y, Qiu X, Chen W, Lin Q, Zeng Y, et al. 2019; A verification of the application of the non-derivatized mass spectrometry method in newborns screening of metabolic disorders. Medicine (Baltimore). 98:e15500. DOI: 10.1097/MD.0000000000015500. PMID: 31083189. PMCID: PMC6531236.
Article6. U.S. Food and Drug Administration. 510(k) Premarket notification (K17 3568). https://www.accessdata.fda.gov/cdrh_docs/reviews/K173568.pdf. Updated on Sept 2018.7. Levy HL, Shih VE, Madigan PM, MacCready RA. 1969; Transient tyrosinemia in full-term infants. JAMA. 209:249–50. DOI: 10.1001/jama.1969.03160150035008. PMID: 5819230.
Article8. Zea-Rey AV, Cruz-Camino H, Vazquez-Cantu DL, Gutiérrez-García VM, Santos-Guzmán J, Cantú-Reyna C. 2017; The incidence of transient neonatal tyrosinemia within a Mexican population. J Inborn Errors Metab Screen. 5:e170016. DOI: 10.1177/2326409817744230.
Article9. Park HD, Lee DH, Choi TY, Lee YK, Kim JW, Ki CS, et al. 2009; Clinical, biochemical, and genetic analysis of a Korean neonate with hereditary tyrosinemia type 1. Clin Chem Lab Med. 47:930–3. DOI: 10.1515/CCLM.2009.223. PMID: 19569981.
Article10. Choi HJ, Bang HI, Ki CS, Lee SY, Kim JW, Song J, et al. 2014; Two novel FAH gene mutations in a patient with hereditary tyrosinemia type I. Ann Clin Lab Sci. 44:317–23.11. Clark RH, Kelleher AS, Chace DH, Spitzer AR. 2014; Gestational age and age at sampling influence metabolic profiles in premature infants. Pediatrics. 134:e37–46. DOI: 10.1542/peds.2014-0329. PMID: 24913786.
Article12. Gucciardi A, Zaramella P, Costa I, Pirillo P, Nardo D, Naturale M, et al. 2015; Analysis and interpretation of acylcarnitine profiles in dried blood spot and plasma of preterm and full-term newborns. Pediatr Res. 77:36–47. DOI: 10.1038/pr.2014.142. PMID: 25268144.
Article13. Liu Q, Wu J, Shen W, Wei R, Jiang J, Liang J, et al. 2017; Analysis of amino acids and acyl carnitine profiles in low birth weight, preterm, and small for gestational age neonates. J Matern Fetal Neonatal Med. 30:2697–704. DOI: 10.1080/14767058.2016.1261395. PMID: 27844490.
Article14. Lee HS. 2021; Arginine, as a key indicator for real-time stability monitoring of quality control in the newborn screening test using dried blood spot. Separations. 8:201. DOI: 10.3390/separations8110201.
Article15. CLSI. 2017. Newborn screening by tandem mass spectrometry. CLSI NBS04. Clinical and Laboratory Standards Institute;Wayne, PA:16. CLSI. 2014. Liquid chromatography-mass spectrometry methods; approved guideline. CLSI C62-A. Clinical and Laboratory Standards Institute;Wayne, PA:17. Moon SY, Choi HJ, Kim S, Lee K, Lee SG, Song SH, et al. 2020; Recommendations for liquid chromatography-mass spectrometry in the clinical laboratory: part II. Method validation. Lab Med Online. 10:95–108. DOI: 10.3343/lmo.2020.10.2.95.
Article18. Wickham H. ggplot2: elegant graphics for data analysis. Use R;2016.19. CLSI. 2010. Defining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline-third edition. CLSI EP28-A3C. Clinical and Laboratory Standards Institute;Wayne, PA:20. Centers for Disease Control and Prevention (CDC). Newborn Screening Quality Assurance Program (NSQAP) 2020 Annual Summary Report. https://www.cdc.gov/labstandards/pdf/nsqap/NSQAP_Annual_Summary_2020-508.pdf. Updated on Aug 2020.21. Lee SY. 2020; Report of the Korean Association of External Quality Assessment Service on metabolite testing (2018-2019). Lab Med Qual Assur. 42:10–25. DOI: 10.15263/jlmqa.2020.42.1.10.
Article22. Cho SE, Park EJ, Seo DH, Lee IB, Lee HJ, Cho DY, et al. 2015; Neonatal screening tests for inherited metabolic disorders using tandem mass spectrometry: experience of a clinical laboratory in Korea. Lab Med Online. 5:196–203. DOI: 10.3343/lmo.2015.5.4.196.
Article23. Chae H, Cho SE, Park HD, Chun S, Lee YW, Yun YM, et al. 2019; Use of liquid chromatography-tandem mass spectrometry for clinical testing in Korean laboratories: a questionnaire survey. Ann Lab Med. 39:447–53. DOI: 10.3343/alm.2019.39.5.447. PMID: 31037863. PMCID: PMC6502944.
Article24. Choi R, Chun MR, Park J, Lee JW, Ju HY, Cho HW, et al. 2021; Quantification of thioguanine in DNA using liquid chromatography-tandem mass spectrometry for routine thiopurine drug monitoring in patients with pediatric acute lymphoblastic leukemia. Ann Lab Med. 41:145–54. DOI: 10.3343/alm.2021.41.2.145. PMID: 33063676. PMCID: PMC7591283.
Article25. Choi R, Park HD, Oh HJ, Lee K, Song J, Lee SY. 2019; Dried blood spot multiplexed steroid profiling using liquid chromatography tandem mass spectrometry in Korean neonates. Ann Lab Med. 39:263–70. DOI: 10.3343/alm.2019.39.3.263. PMID: 30623618. PMCID: PMC6340850.
Article26. Hall PL, Marquardt G, McHugh DM, Currier RJ, Tang H, Stoway SD, et al. 2014; Postanalytical tools improve performance of newborn screening by tandem mass spectrometry. Genet Med. 16:889–95. DOI: 10.1038/gim.2014.62. PMID: 24875301. PMCID: PMC4262759.
Article27. Saudubray J-M, van den Berghe G, Walter JHW. 2012. Inborn metabolic diseases: diagnosis and treatment. 5th ed. Springer;Berlin: p. 656.28. Levy HL, Shih VE, Madigan PM. 1974; Routine newborn screening for histidinemia. Clinical and biochemical results. N Engl J Med. 291:1214–9. DOI: 10.1056/NEJM197412052912303. PMID: 4421298.29. Lam WK, Cleary MA, Wraith JE, Walter JH. 1996; Histidinaemia: a benign metabolic disorder. Arch Dis Child. 74:343–6. DOI: 10.1136/adc.74.4.343. PMID: 8669938. PMCID: PMC1511463.
Article30. Rappold BA. 2022; Review of the use of liquid chromatography-tandem mass spectrometry in clinical laboratories: part I-development. Ann Lab Med. 42:121–40. DOI: 10.3343/alm.2022.42.2.121. PMID: 34635606. PMCID: PMC8548246.
Article31. Pickens CA, Sternberg M, Seeterlin M, De Jesús VR, Morrissey M, Manning A, et al. 2020; Harmonizing newborn screening laboratory proficiency test results using the CDC NSQAP reference materials. Int J Neonatal Screen. 6:75. DOI: 10.3390/ijns6030075. PMID: 33123642. PMCID: PMC7570198.
Article32. CLSI. 2019. Newborn screening for preterm, low birth weight, and sick newborns; approved guideline. 2nd ed. CLSI NBS03-A. Clinical and Laboratory Standards Institute;Wayne, PA:33. Panfoli I, Cassanello M, Bruschettini M, Colella M, Cerone R, Ravera S, et al. 2016; Why do premature newborn infants display elevated blood adenosine levels? Med Hypotheses. 90:53–6. DOI: 10.1016/j.mehy.2016.03.007. PMID: 27063086.
Article34. Peng G, Tang Y, Cowan TM, Zhao H, Scharfe C. 2020; Timing of newborn blood collection alters metabolic disease screening performance. Front Pediatr. 8:623184. DOI: 10.3389/fped.2020.623184. PMID: 33553077. PMCID: PMC7854909.
Article35. De Jesús VR, Adam BW, Mandel D, Cuthbert CD, Matern D. 2014; Succinylacetone as primary marker to detect tyrosinemia type I in newborns and its measurement by newborn screening programs. Mol Genet Metab. 113:67–75. DOI: 10.1016/j.ymgme.2014.07.010. PMID: 25066104. PMCID: PMC4533100.
Article36. Flinn AM, Gennery AR. 2018; Adenosine deaminase deficiency: a review. Orphanet J Rare Dis. 13:65. DOI: 10.1186/s13023-018-0807-5. PMID: 29690908. PMCID: PMC5916829.
Article37. Turgeon CT, Moser AB, Mørkrid L, Magera MJ, Gavrilov DK, Oglesbee D, et al. 2015; Streamlined determination of lysophosphatidylcholines in dried blood spots for newborn screening of X-linked adrenoleukodystrophy. Mol Genet Metab. 114:46–50. DOI: 10.1016/j.ymgme.2014.11.013. PMID: 25481105.
Article38. Mashima R, Tanaka M, Sakai E, Nakajima H, Kumagai T, Kosuga M, et al. 2016; A selective detection of lysophosphatidylcholine in dried blood spots for diagnosis of adrenoleukodystrophy by LC-MS/MS. Mol Genet Metab Rep. 7:16–9. DOI: 10.1016/j.ymgmr.2016.02.007. PMID: 27331004. PMCID: PMC4908058.
Article39. Lee S, Clinard K, Young SP, Rehder CW, Fan Z, Calikoglu AS, et al. 2020; Evaluation of X-linked adrenoleukodystrophy newborn screening in North Carolina. JAMA Netw Open. 3:e1920356. DOI: 10.1001/jamanetworkopen.2019.20356. PMID: 32003821. PMCID: PMC7042889.
Article40. Adam BW, Hall EM, Meredith NK, Lim TH, Haynes CA, De Jesus VR, et al. 2012; Performance of succinylacetone assays and their associated proficiency testing outcomes. Clin Biochem. 45:1658–63. DOI: 10.1016/j.clinbiochem.2012.08.007. PMID: 22906829. PMCID: PMC4557813.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of the serum erythropoietin levels in neonates
- Umbilical Cord Arterial Concentrations of Isoprostane(8-iso-PGF2alpha) in Newborn Infants
- Hypercalciuria in High Risk Neonates
- Gastric Perforation in the Neonatal Period: Differences between Preterm and Term Infants
- The Utility of Serum Prealbumin as a Biochemical Marker for Nutritional Adequacy in Neonates