J Stroke.
2024 Jan;26(1):64-74. 10.5853/jos.2023.02733.
Long-Term Resveratrol Intake for Cognitive and Cerebral Blood Flow Impairment in Carotid Artery Stenosis/Occlusion
- Affiliations
-
- 1Department of Neurology, National Cerebral and Cardiovascular Center, Suita, Japan
- 2Department of Pharmacology, National Cerebral and Cardiovascular Center, Suita, Japan
- 3R&D Division, Towa Pharmaceutical Co., Ltd., Kadoma, Japan
- 4Turku PET Centre, University of Turku, Turku, Finland
- KMID: 2551348
- DOI: http://doi.org/10.5853/jos.2023.02733
Abstract
- Background and Purpose
Carotid artery stenosis or occlusion (CASO) is a causative disease of vascular cognitive impairment (VCI) attributed to cerebral hypoperfusion, even without the development of symptomatic ischemic stroke. Preclinically, resveratrol has been demonstrated to play an important role in improving cognitive function in rodent CASO models. This study investigated the association between long-term resveratrol intake and improvements in cognitive and cerebral hemodynamic impairments in patients with CASO.
Methods
A retrospective cohort study was conducted on patients with asymptomatic carotid artery stenosis of ≥50% or occlusion who underwent 15O-gas positron emission tomography (15O-gas PET) and neuropsychological tests such as Montreal Cognitive Assessment (MoCA) and Alzheimer’s Disease Assessment Scale-Cognitive Subscale 13 (ADAS-Cog) twice between July 2020 and March 2022 allowing >125-day interval. Patients were administered 30 mg/day resveratrol after the first 15O-gas PET and neuropsychological tests were compared with those who were not.
Results
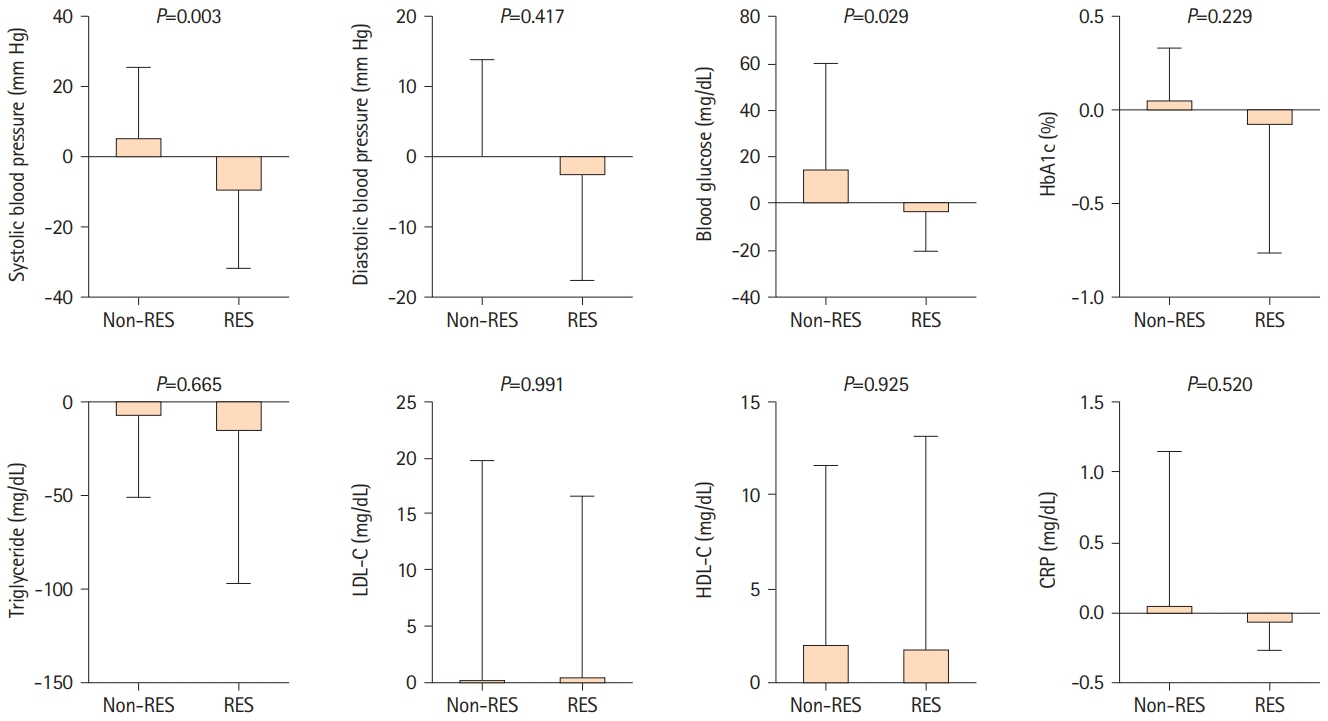
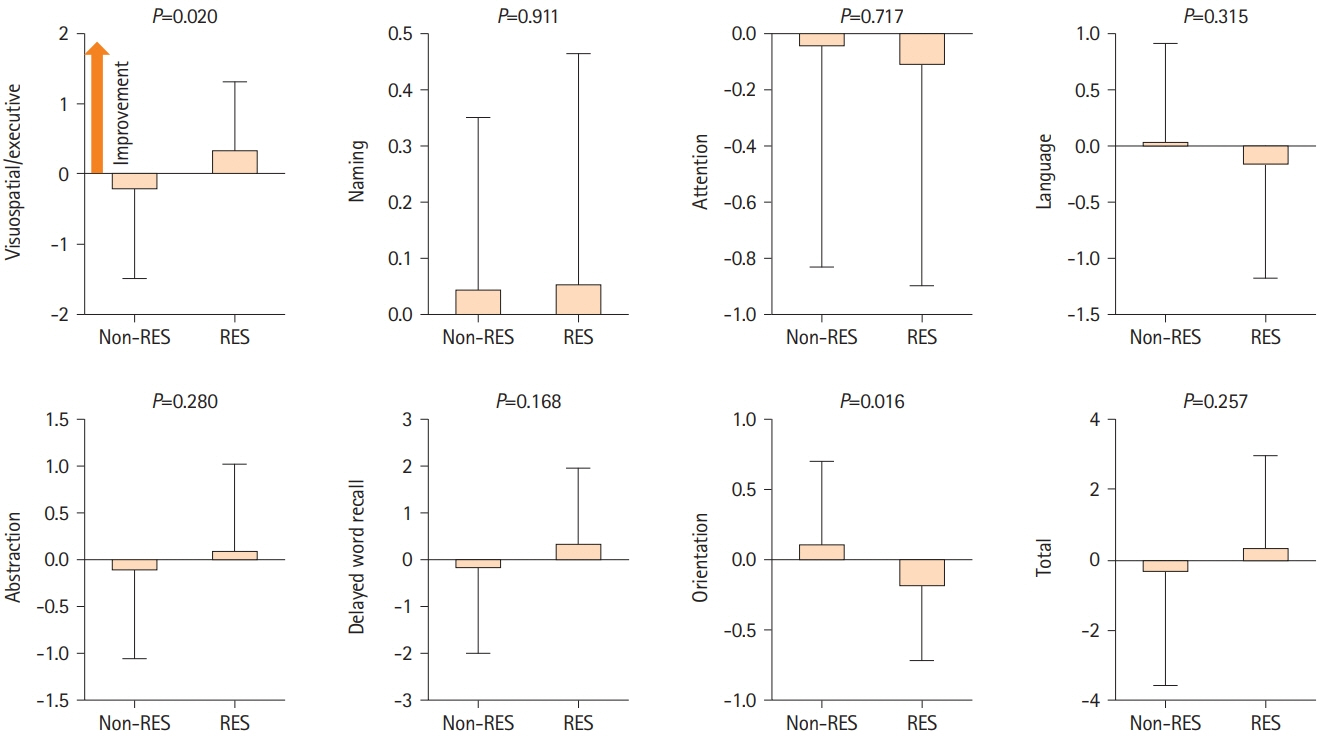
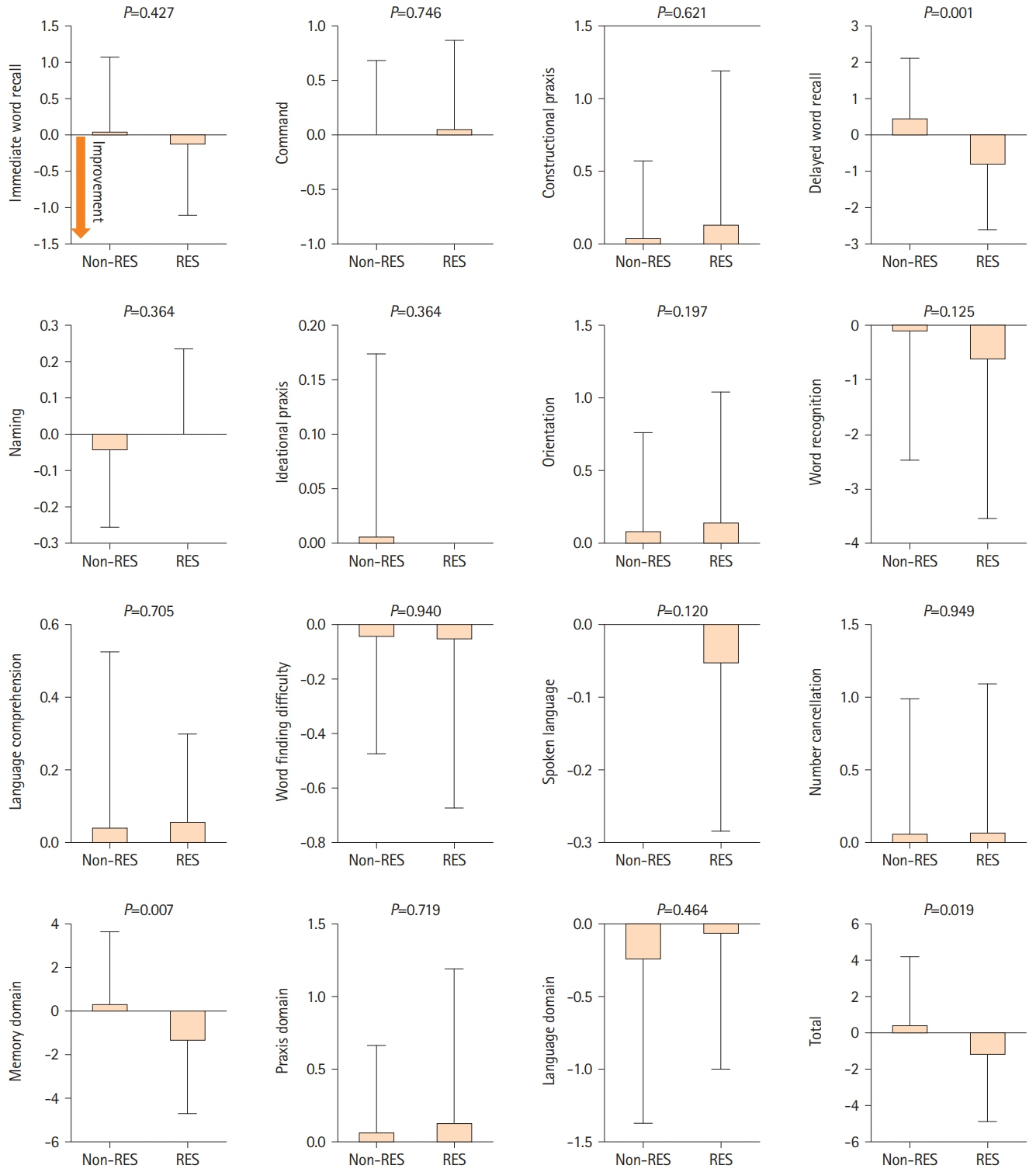
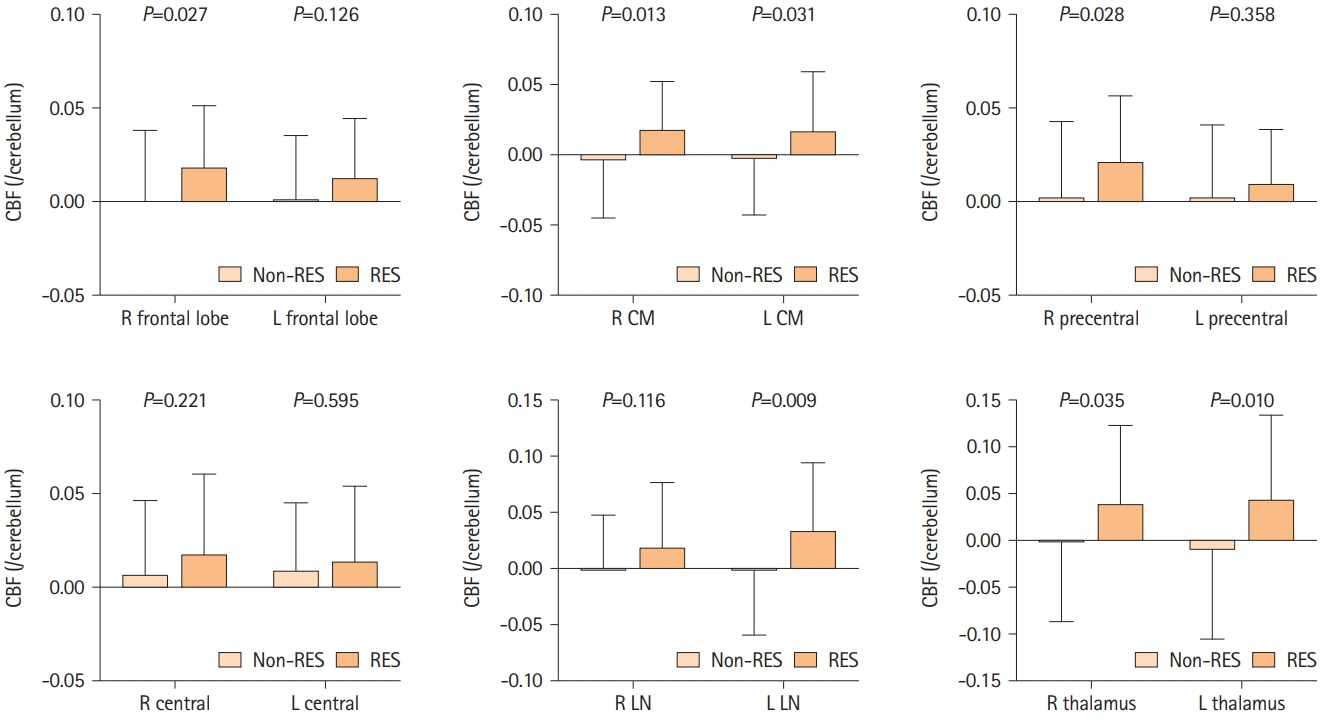
A total of 79 patients were enrolled in this study; 36 received resveratrol and 43 did not. Over a mean follow-up of 221.2 and 244.8 days, long-term resveratrol treatment significantly improved visuospatial/executive function (P=0.020) in MoCA, and memory domain (P=0.007) and total score (P=0.019) in ADAS-Cog. Cerebral blood flow demonstrated improvement in the right frontal lobe (P=0.027), left lenticular nucleus (P=0.009), right thalamus (P=0.035), and left thalamus (P=0.010) on 15O-gas PET. No adverse events were reported.
Conclusion
Long-term daily intake of oral resveratrol may prevent or treat VCI by improving the cerebral blood flow in asymptomatic patients with CASO.
Keyword
Figure
Reference
-
References
1. Balestrini S, Perozzi C, Altamura C, Vernieri F, Luzzi S, Bartolini M, et al. Severe carotid stenosis and impaired cerebral hemodynamics can influence cognitive deterioration. Neurology. 2013; 80:2145–2150.2. Silvestrini M, Paolino I, Vernieri F, Pedone C, Baruffaldi R, Gobbi B, et al. Cerebral hemodynamics and cognitive performance in patients with asymptomatic carotid stenosis. Neurology. 2009; 72:1062–1068.3. Nickel A, Kessner S, Niebuhr A, Schröder J, Malherbe C, Fischer F, et al. Cortical thickness and cognitive performance in asymptomatic unilateral carotid artery stenosis. BMC Cardiovasc Disord. 2019; 19:154.4. Lal BK, Dux MC, Sikdar S, Goldstein C, Khan AA, Yokemick J, et al. Asymptomatic carotid stenosis is associated with cognitive impairment. J Vasc Surg. 2017; 66:1083–1092.5. Witte AV, Kerti L, Margulies DS, Flöel A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J Neurosci. 2014; 34:7862–7870.6. Griñán-Ferré C, Bellver-Sanchis A, Izquierdo V, Corpas R, Roig-Soriano J, Chillón M, et al. The pleiotropic neuroprotective effects of resveratrol in cognitive decline and Alzheimer’s disease pathology: from antioxidant to epigenetic therapy. Ageing Res Rev. 2021; 67:101271.7. Wang N, He J, Pan C, Wang J, Ma M, Shi X, et al. Resveratrol activates autophagy via the AKT/mTOR signaling pathway to improve cognitive dysfunction in rats with chronic cerebral hypoperfusion. Front Neurosci. 2019; 13:859.8. Li H, Wang J, Wang P, Rao Y, Chen L. Resveratrol reverses the synaptic plasticity deficits in a chronic cerebral hypoperfusion rat model. J Stroke Cerebrovasc Dis. 2016; 25:122–128.9. Kang N, Shi Y, Song J, Gao F, Fan M, Jin W, et al. Resveratrol reduces inflammatory response and detrimental effects in chronic cerebral hypoperfusion by down-regulating stimulator of interferon genes/TANK-binding kinase 1/interferon regulatory factor 3 signaling. Front Aging Neurosci. 2022; 14:868484.10. Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012; 148:421–433.11. Hattori Y, Okamoto Y, Maki T, Yamamoto Y, Oishi N, Yamahara K, et al. Silent information regulator 2 homolog 1 counters cerebral hypoperfusion injury by deacetylating endothelial nitric oxide synthase. Stroke. 2014; 45:3403–3411.12. Hattori Y, Okamoto Y, Nagatsuka K, Takahashi R, Kalaria RN, Kinoshita M, et al. SIRT1 attenuates severe ischemic damage by preserving cerebral blood flow. Neuroreport. 2015; 26:113–117.13. Evans HM, Howe PRC, Wong RHX. Effects of resveratrol on cognitive performance, mood and cerebrovascular function in post-menopausal women; a 14-week randomised placebo-controlled intervention trial. Nutrients. 2017; 9:27.14. Gordon DL. The diagnosis and management of cerebral venous thrombosis. In: Adams HP. Handbook of Cerebrovascular Diseases, Revised and Expanded. 2nd ed. Boca Raton, FL: CRC Press;2004. p. 605–635.15. Jahromi AS, Cinà CS, Liu Y, Clase CM. Sensitivity and specificity of color duplex ultrasound measurement in the estimation of internal carotid artery stenosis: a systematic review and meta-analysis. J Vasc Surg. 2005; 41:962–972.16. Lazar RM, Wadley VG, Myers T, Jones MR, Heck DV, Clark WM, et al. Baseline cognitive impairment in patients with asymptomatic carotid stenosis in the CREST-2 trial. Stroke. 2021; 52:3855–3863.17. Bonati LH, Kakkos S, Berkefeld J, de Borst GJ, Bulbulia R, Halliday A, et al. European Stroke Organisation guideline on endarterectomy and stenting for carotid artery stenosis. Eur Stroke J. 2021; 6:I–XLVII.18. Iguchi S, Moriguchi T, Yamazaki M, Hori Y, Koshino K, Toyoda K, et al. System evaluation of automated production and inhalation of 15O-labeled gaseous radiopharmaceuticals for the rapid 15O-oxygen PET examinations. EJNMMI Phys. 2018; 5:37.19. Kudomi N, Hayashi T, Teramoto N, Watabe H, Kawachi N, Ohta Y, et al. Rapid quantitative measurement of CMRO(2) and CBF by dual administration of 15O-labeled oxygen and water during a single PET scan - a validation study and error analysis in anesthetized monkeys. J Cereb Blood Flow Metab. 2005; 25:1209–1224.20. Kudomi N, Choi E, Yamamoto S, Watabe H, Kim KM, Shidahara M, et al. Development of a GSO detector assembly for a continuous blood sampling system. IEEE Trans Nucl Sci. 2003; 50:70–73.21. Iida H, Jones T, Miura S. Modeling approach to eliminate the need to separate arterial plasma in oxygen-15 inhalation positron emission tomography. J Nucl Med. 1993; 34:1333–1340.22. Nishimiya M, Matsuda H, Imabayashi E, Kuji I, Sato N. Comparison of SPM and NEUROSTAT in voxelwise statistical analysis of brain SPECT and MRI at the early stage of Alzheimer’s disease. Ann Nucl Med. 2008; 22:921–927.23. Sato S, Kojima D, Shimada Y, Yoshida J, Fujimato K, Fujiwara S, et al. Preoperatively reduced cerebrovascular contractile reactivity to hypocapnia by hyperventilation is associated with cerebral hyperperfusion syndrome after arterial bypass surgery for adult patients with cerebral misery perfusion due to ischemic moyamoya disease. J Cereb Blood Flow Metab. 2018; 38:1021–1031.24. Saura H, Ogasawara K, Beppu T, Yoshida K, Kobayashi M, Yoshida K, et al. Hypoxic viable tissue in human chronic cerebral ischemia because of unilateral major cerebral artery steno-occlusive disease. Stroke. 2015; 46:1250–1256.25. Miyoshi K, Chida K, Kobayashi M, Kubo Y, Yoshida K, Terasaki K, et al. Two-year clinical, cerebral hemodynamic, and cognitive outcomes of adult patients undergoing medication alone for symptomatically ischemic moyamoya disease without cerebral misery perfusion: a prospective cohort study. Neurosurgery. 2019; 84:1233–1241.26. Podhorna J, Krahnke T, Shear M, E Harrison J. Alzheimer’s Disease Assessment Scale-Cognitive subscale variants in mild cognitive impairment and mild Alzheimer’s disease: change over time and the effect of enrichment strategies. Alzheimers Res Ther. 2016; 8:8.27. Kennedy DO, Wightman EL, Reay JL, Lietz G, Okello EJ, Wilde A, et al. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: a double-blind, placebo-controlled, crossover investigation. Am J Clin Nutr. 2010; 91:1590–1597.28. Yu C, Shin YG, Chow A, Li Y, Kosmeder JW, Lee YS, et al. Human, rat, and mouse metabolism of resveratrol. Pharm Res. 2002; 19:1907–1914.29. Bogousslavsky J, Regli F, Uske A. Thalamic infarcts: clinical syndromes, etiology, and prognosis. Neurology. 1988; 38:837–848.30. Gaunt ME, Martin PJ, Smith JL, Rimmer T, Cherryman G, Ratliff DA, et al. Clinical relevance of intraoperative embolization detected by transcranial Doppler ultrasonography during carotid endarterectomy: a prospective study of 100 patients. Br J Surg. 1994; 81:1435–1439.31. De Rango P, Caso V, Leys D, Paciaroni M, Lenti M, Cao P. The role of carotid artery stenting and carotid endarterectomy in cognitive performance: a systematic review. Stroke. 2008; 39:3116–3127.32. Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011; 42:2672–2713.33. Toth P, Tarantini S, Tucsek Z, Ashpole NM, Sosnowska D, Gautam T, et al. Resveratrol treatment rescues neurovascular coupling in aged mice: role of improved cerebromicrovascular endothelial function and downregulation of NADPH oxidase. Am J Physiol Heart Circ Physiol. 2014; 306:H299–H308.34. Parsamanesh N, Asghari A, Sardari S, Tasbandi A, Jamialahmadi T, Xu S, et al. Resveratrol and endothelial function: a literature review. Pharmacol Res. 2021; 170:105725.35. Wong RHX, Howe PRC, Buckley JD, Coates AM, Kunz I, Berry NM. Acute resveratrol supplementation improves flow-mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutr Metab Cardiovasc Dis. 2011; 21:851–856.36. Wong RHX, Howe PRC. Resveratrol counteracts insulin resistance—potential role of the circulation. Nutrients. 2018; 10:1160.37. Wischik CM, Staff RT, Wischik DJ, Bentham P, Murray AD, Storey JMD, et al. Tau aggregation inhibitor therapy: an exploratory phase 2 study in mild or moderate Alzheimer’s disease. J Alzheimers Dis. 2015; 44:705–720.38. Hayashi E, Iida H, Fukuda T, Iida A. Accuracy of non-invasive arterial input functions and error in quantitative images in the cerebellar reference method. Kaku Igaku. 2021; 58:19–32.39. Goetti R, Warnock G, Kuhn FP, Guggenberger R, O’Gorman R, Buck A, et al. Quantitative cerebral perfusion imaging in children and young adults with Moyamoya disease: comparison of arterial spin-labeling-MRI and H2[15O]-PET. AJNR Am J Neuroradiol. 2014; 35:1022–1028.40. Hara S, Tanaka Y, Ueda Y, Hayashi S, Inaji M, Ishiwata K, et al. Noninvasive evaluation of CBF and perfusion delay of moyamoya disease using arterial spin-labeling MRI with multiple postlabeling delays: comparison with 15O-Gas PET and DSC-MRI. AJNR Am J Neuroradiol. 2017; 38:696–702.41. Smailagic N, Vacante M, Hyde C, Martin S, Ukoumunne O, Sachpekidis C. 18F-FDG PET for the early diagnosis of Alzheimer’s disease dementia and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev. 2015; 1:CD010632.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cerebral Vasoreactivity in Carotid Stenosis or Occlusion Cases: A Transcranial Doppler Study with Acetazolamide (Diamox) Test
- Spectral Analysis of EEG with Reversible Middle Cerebral Artery Occlusion in Rats
- Regional Cerebral Blood Flow Changes in Traumatic Carotid Cavernous Fistula During Trapping Procedure: Case Study, Preliminary Report
- External Carotid Artery Angioplasty and Stenting Followed by Superficial Temporal Artery to Middle Cerebral Artery Anastomosis
- Multiple Carotid Artery Occlusive Diseases Treated with Staged Subclavian-carotid Artery bypass and Carotid Endarterectomy: Case Report