Ann Clin Microbiol.
2023 Sep;26(3):51-57. 10.5145/ACM.2023.26.3.2.
Introduction to the revised international guidelines on breakpoints for antimicrobial susceptibility testing
- Affiliations
-
- 1Department of Laboratory Medicine, Dongguk University Ilsan Hospital, Goyang, Korea
- KMID: 2550655
- DOI: http://doi.org/10.5145/ACM.2023.26.3.2
Abstract
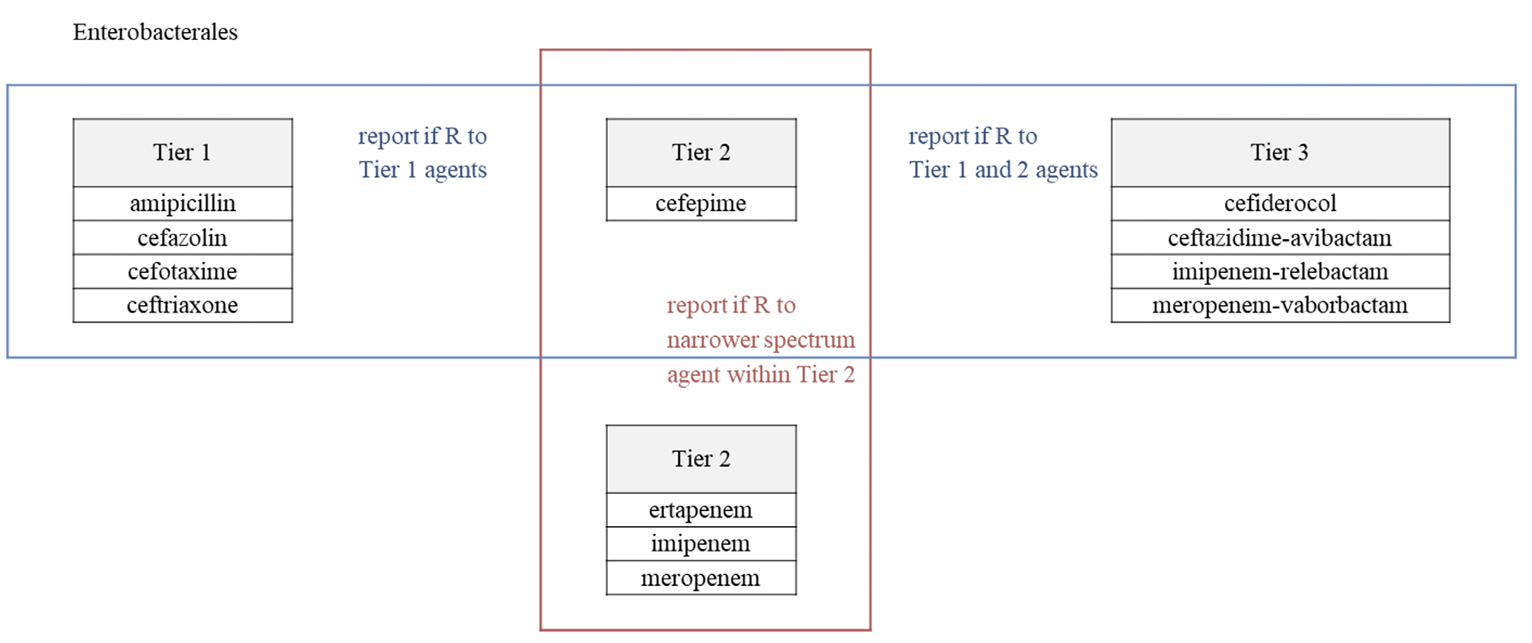
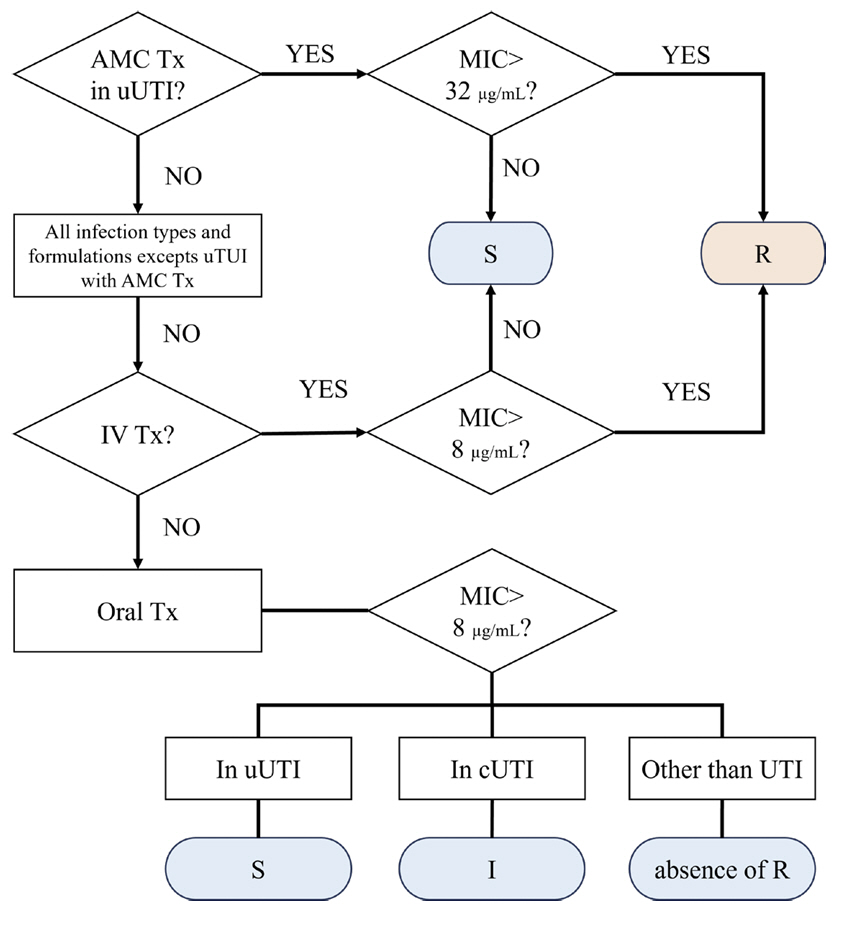
- Clinical and Laboratory Standards Institute (CLSI) M100 ‘Performance Standards for Antimicrobial Susceptibility Testing (AST)’ and the European Committee on AST (EUCAST) ‘Breakpoint tables for interpretation of MICs and zone diameters’ guidelines for conducting and interpreting AST are revised yearly. The 2023 CLSI guideline introduces selective and cascade reporting methods for antibacterial agents as a part of strengthening antibiotic stewardship and changes in breakpoints for aminoglycoside (AG) in Enterobacterales and AG and piperacillin in Pseudomonas aeruginosa. Main changes in EUCAST include revised breakpoints for aminopenicillins in Enterobacterales, and detailed criteria reflecting the clinical situation and antibacterial agent administration method.
Keyword
Figure
Reference
-
1. CLSI. Performance Standards for Antimicrobial Susceptibility Testing. CLSI supplement M100. 33rd ed, Wayne, PA; Clinical and Laboratory Standards Institute, 2023. .2. Chang CL. Introduction of Clinical and Laboratory Standards Institute antibiotic susceptibility testing subcommittee meeting. Ann Clin Microbiol 2018;21:69-74. .3. Sader HS, Mendes RE, Kimbrough JH, Kantro V, Castanheira M. Impact of the recent Clinical and Laboratory Standards Institute breakpoint changes on the antimicrobial spectrum of aminoglycosides and the activity of plazomicin against multidrug-resistant and carbapenemresistant Enterobacterales from United States medical centers. Open Forum Infect Dis 2023;10:ofad058. .4. Castanheira M, Sader HS, Mendes RE, Jones RN. Activity of plazomicin tested against Enterobacterales isolates collected from U.S. hospitals in 2016-2017: effect of different breakpoint criteria on susceptibility rates among aminoglycosides. Antimicrob Agents Chemother 2020;64:10-1128. .5. CLSI. Performance Standards for Antimicrobial Susceptibility Testing. CLSI supplement M100. 32nd ed, Wayne, PA; Clinical and Laboratory Standards Institute, 2022. .6. The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 12.0. https://www.eucast.org/fileadmin/src/ media/PDFs/EUCAST_files/Breakpoint_tables/v_12.0_Breakpoint_Tables.pdf [Online] (last visited on 4 September 2023). .7. Yamagishi Y, Terada M, Ohki E, Miura Y, Umemura T, Mikamo H. Investigation of the clinical breakpoints of piperacillin-tazobactam against infections caused by Pseudomonas aeruginosa. J Infect Chemother 2012;18:127-9. .8. Wu H, Lutgring JD, McDonald LC, Webb A, Fields V, Blum L, et al. Selective and cascade reporting of antimicrobial susceptibility testing results and its impact on antimicrobial resistance surveillance-national healthcare safety network, April 2020 to March 2021. Microbiol Spectr 2023;11:e0164622. .9. The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 13.1. https://www.eucast.org/fileadmin/src/ media/PDFs/EUCAST_files/Breakpoint_tables/v_13.1_Breakpoint_Tables.pdf [Online] (last visited on 4 September 2023). .10. de Velde F, de Winter BC, Koch BC, van Gelder T, Mouton JW. Non-linear absorption pharmacokinetics of amoxicillin: consequences for dosing regimens and clinical breakpoints. J Antimicrob Chemother 2016;71:2909-17. .
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- EUCAST v.12.0 review focused on changes
- Impact of Revised Penicillin Breakpoints for Streptococcus pneumoniae (CLSI M100-S18) on the Penicillin Susceptibility Rate
- Pitfalls of the Clinical and Laboratory Standards Institute’s Revised Breakpoints on Interpretation of the Cephalosporin Susceptibility of an Extended-Spectrum β-lactamase Producing Klebsiella pneumoniae: Analysis of a 2010 Nationwide Proficiency Survey
- Strategies for Interpretive Standards of beta-Lactams Susceptibility Testing and Identification of Extended-Spectrum beta-Lactamases and Carbapenemases in Enterobacteriaceae
- Comparison of Clinical and Laboratory Standards Institute and European Committee on Antimicrobial Susceptibility Testing Breakpoints for β-Lactams in Enterobacteriaceae Producing Extended-Spectrum β-Lactamases and/or Plasmid-Mediated AmpC β-Lactamases