J Pathol Transl Med.
2024 Jan;58(1):29-34. 10.4132/jptm.2023.12.07.
Immunohistochemical expression of anaplastic lymphoma kinase in neuroblastoma and its relations with some clinical and histopathological features
- Affiliations
-
- 1Department of Pathology, University of Medicine and Pharmacy at Ho Chi Minh City, Ho Chi Minh City, Vietnam
- 2Department of Oncology–Hematology, Children Hospital 2, Ho Chi Minh City, Vietnam
- KMID: 2550498
- DOI: http://doi.org/10.4132/jptm.2023.12.07
Abstract
- Background
Anaplastic lymphoma kinase (ALK) mutations have been identified as a prominent cause of some familial and sporadic neuroblastoma (NB). ALK expression in NB and its relationship with clinical and histopathological features remains controversial. This study investigated ALK expression and its potential relations with these features in NB.
Methods
Ninety cases of NB at the Department of Pathology, University of Medicine and Pharmacy at Ho Chi Minh City, Viet Nam from 01/01/2018 to 12/31/2021, were immunohistochemically stained with ALK (D5F3) antibody. The ALK expression and its relations with some clinical and histopathological features were investigated.
Results
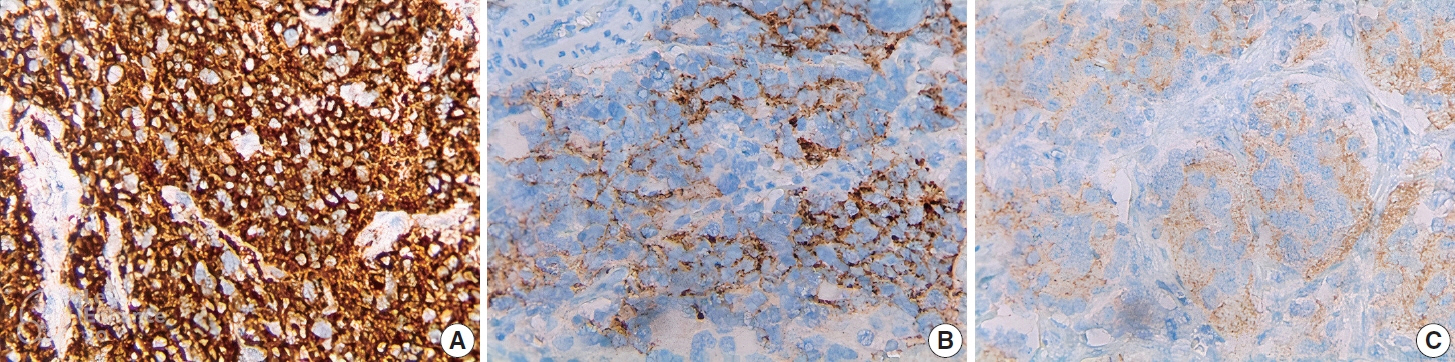
The rate of ALK expression in NB was 91.1%. High ALK expression (over 50% of tumor cells were positive with moderate-strong intensity) accounted for 65.6%, and low ALK expression accounted for 34.4%. All the MYCN-amplified NB patients had ALK immunohistochemistry positivity, most cases had high ALK protein expression. The undifferentiated subtype of NB had a lower ALK-positive rate than the poorly differentiated and differentiated subtype. The percentages of ALK positivity were significantly higher in more differentiated histological types of NB (p = .024). There was no relation between ALK expression and: age group, sex, primary tumor location, tumor stage, MYCN status, clinical risk, Mitotic-Karyorrhectic Index, prognostic group, necrosis, and calcification.
Conclusions
ALK was highly expressed in NB. ALK expression was not related to several clinical and histopathological features. More studies are needed to elucidate the association between ALK expression and ALK gene status and to investigate disease progression, especially the oncogenesis of ALK-positive NB.
Keyword
Figure
Reference
-
References
1. Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007; 369:2106–20.2. Passoni L, Longo L, Collini P, et al. Mutation-independent anaplastic lymphoma kinase overexpression in poor prognosis neuroblastoma patients. Cancer Res. 2009; 69:7338–46.3. Pugh TJ, Morozova O, Attiyeh EF, et al. The genetic landscape of high-risk neuroblastoma. Nat Genet. 2013; 45:279–84.4. Carpenter EL, Haglund EA, Mace EM, et al. Antibody targeting of anaplastic lymphoma kinase induces cytotoxicity of human neuroblastoma. Oncogene. 2012; 31:4859–67.5. Duijkers FA, Gaal J, Meijerink JP, et al. High anaplastic lymphoma kinase immunohistochemical staining in neuroblastoma and ganglioneuroblastoma is an independent predictor of poor outcome. Am J Pathol. 2012; 180:1223–31.6. Wang M, Zhou C, Sun Q, et al. ALK amplification and protein expression predict inferior prognosis in neuroblastomas. Exp Mol Pathol. 2013; 95:124–30.7. Lee JW, Park SH, Kang HJ, Park KD, Shin HY, Ahn HS. ALK protein expression is related to neuroblastoma aggressiveness but is not independent prognostic factor. Cancer Res Treat. 2018; 50:495–505.8. Yan B, Kuick CH, Lim M, et al. Platform comparison for evaluation of ALK protein immunohistochemical expression, genomic copy number and hotspot mutation status in neuroblastomas. PLoS One. 2014; 9:e106575.9. Sano R, Krytska K, Larmour CE, et al. An antibody-drug conjugate directed to the ALK receptor demonstrates efficacy in preclinical models of neuroblastoma. Sci Transl Med. 2019; 11:eaau9732.10. Aygun Z, Batur S, Emre S, Celkan T, Ozman O, Comunoglu N. Frequency of ALK and GD2 expression in neuroblastoma. Fetal Pediatr Pathol. 2019; 38:326–34.11. Chang HH, Lu MY, Yang YL, et al. The prognostic roles of and correlation between ALK and MYCN protein expression in neuroblastoma. J Clin Pathol. 2020; 73:154–61.12. De Brouwer S, De Preter K, Kumps C, et al. Meta-analysis of neuroblastomas reveals a skewed ALK mutation spectrum in tumors with MYCN amplification. Clin Cancer Res. 2010; 16:4353–62.13. Uryu K, Nishimura R, Kataoka K, et al. Identification of the genetic and clinical characteristics of neuroblastomas using genome-wide analysis. Oncotarget. 2017; 8:107513–29.14. Kim EK, Kim S. ALK gene copy number gain and immunohistochemical expression status using three antibodies in neuroblastoma. Pediatr Dev Pathol. 2017; 20:133–41.15. Schonherr C, Ruuth K, Kamaraj S, et al. Anaplastic lymphoma kinase (ALK) regulates initiation of transcription of MYCN in neuroblastoma cells. Oncogene. 2012; 31:5193–200.16. Wulf AM, Moreno MM, Paka C, Rampasekova A, Liu KJ. Defining pathological activities of ALK in neuroblastoma, a neural crest-derived cancer. Int J Mol Sci. 2021; 22:11718.17. Nakazawa A. Biological categories of neuroblastoma based on the international neuroblastoma pathology classification for treatment stratification. Pathol Int. 2021; 71:232–44.18. Ilie MI, Bence C, Hofman V, et al. Discrepancies between FISH and immunohistochemistry for assessment of the ALK status are associated with ALK ‘borderline’-positive rearrangements or a high copy number: a potential major issue for anti-ALK therapeutic strategies. Ann Oncol. 2015; 26:238–44.19. Nakamura H, Tsuta K, Yoshida A, et al. Aberrant anaplastic lymphoma kinase expression in high-grade pulmonary neuroendocrine carcinoma. J Clin Pathol. 2013; 66:705–7.20. Akhoundova D, Haberecker M, Fritsch R, et al. Targeting ALK in neuroendocrine tumors of the lung. Front Oncol. 2022; 12:911294.21. Leal JL, Peters G, Szaumkessel M, et al. NTRK and ALK rearrangements in malignant pleural mesothelioma, pulmonary neuroendocrine tumours and non-small cell lung cancer. Lung Cancer. 2020; 146:154–9.22. Zheng Q, Zheng M, Jin Y, et al. ALK-rearrangement neuroendocrine carcinoma of the lung: a comprehensive study of a rare case series and review of literature. Onco Targets Ther. 2018; 11:4991–8.23. Sun JM, Choi YL, Won JK, et al. A dramatic response to crizotinib in a non-small-cell lung cancer patient with IHC-positive and FISHnegative ALK. J Thorac Oncol. 2012; 7:e36–8.24. van der Wekken AJ, Pelgrim R, t Hart N, et al. Dichotomous ALKIHC is a better predictor for ALK inhibition outcome than traditional ALK-FISH in advanced non-small cell lung cancer. Clin Cancer Res. 2017; 23:4251–8.25. Brenner AK, Gunnes MW. Therapeutic targeting of the anaplastic lymphoma kinase (ALK) in neuroblastoma: a comprehensive update. Pharmaceutics. 2021; 13:1427.26. Kozuma Y, Toyokawa G, Seto T. ALK testing methods: is there a winner or loser? Expert Rev Anticancer Ther. 2019; 19:237–44.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anaplastic Lymphoma Kinase-Positive Anaplastic Large Cell Lymphoma Arising in a Patient with Hypersensitivity to Mosquito Bites
- CD30-Positive Anaplastic Lymphoma Kinase-Negative Systemic Anaplastic Large-Cell Lymphoma in a 9-Year-Old Boy
- A Case of Multiple Cranial Neuropathies Caused by Anaplastic Lymphoma Kinase-Negative Anaplastic Large Cell Lymphoma
- ALK Protein Expression Is Related to Neuroblastoma Aggressiveness But Is Not Independent Prognostic Factor
- Oral spindle cell/sclerosing rhabdomyosarcoma on mandible with anaplastic lymphoma kinase expression mimicking inflammatory myofibroblastic tumor