Ann Lab Med.
2024 Jan;44(1):64-73. 10.3343/alm.2024.44.1.64.
Association Between Low Anti-spike Antibody Levels After the Third Dose of SARS-CoV-2 Vaccination and Hospitalization due to Symptomatic Breakthrough Infection in Kidney Transplant Recipients
- Affiliations
-
- 1Department of Surgery, Seoul National University College of Medicine, Seoul, Korea
- 2Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
- 3Department of Pediatrics, Seoul National University College of Medicine, Seoul, Korea
- 4Department of Laboratory Medicine, Seoul National University College of Medicine, Seoul, Korea
- 5Transplantation Research Institute, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2550224
- DOI: http://doi.org/10.3343/alm.2024.44.1.64
Abstract
- Background
Whether anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody levels post-third coronavirus disease (COVID-19) vaccination correlate with worse outcomes due to breakthrough infection is unclear. We evaluated the association between anti-SARS-CoV-2 antibody levels and symptomatic breakthrough infection or hospitalization during the Omicron surge in kidney transplant recipients.
Methods
In total, 287 kidney transplant recipients expected to receive a third vaccination were enrolled between November 2021 and February 2022. The Abbott SARS-CoV-2 IgG II Quant test (Abbott, Chicago, IL, USA) was performed within three weeks before and four weeks after the third vaccination. The incidence of symptomatic breakthrough infection and hospitalization from two weeks to four months post-third vaccination was recorded.
Results
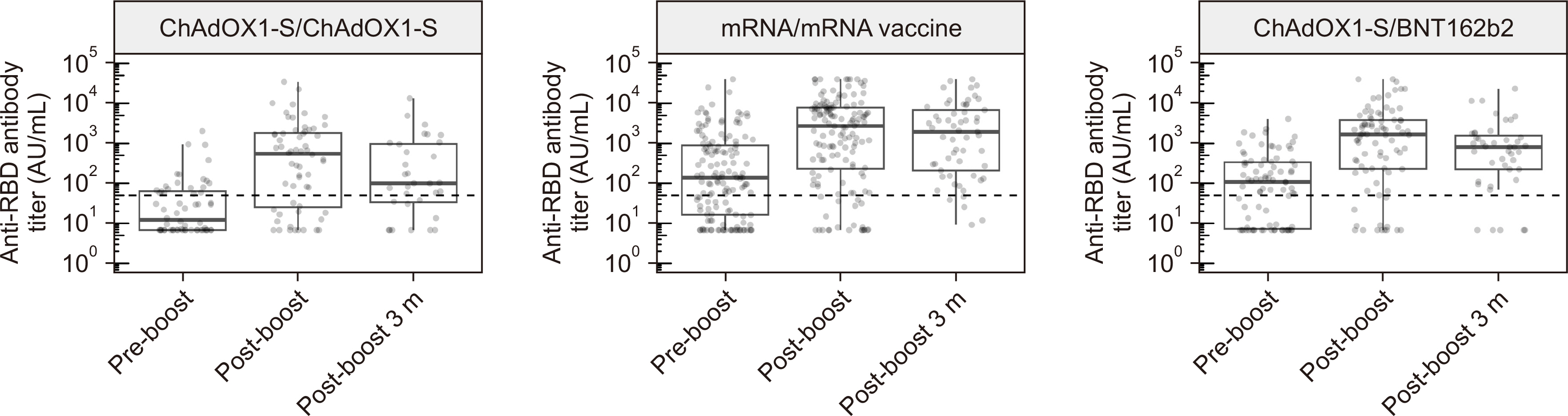
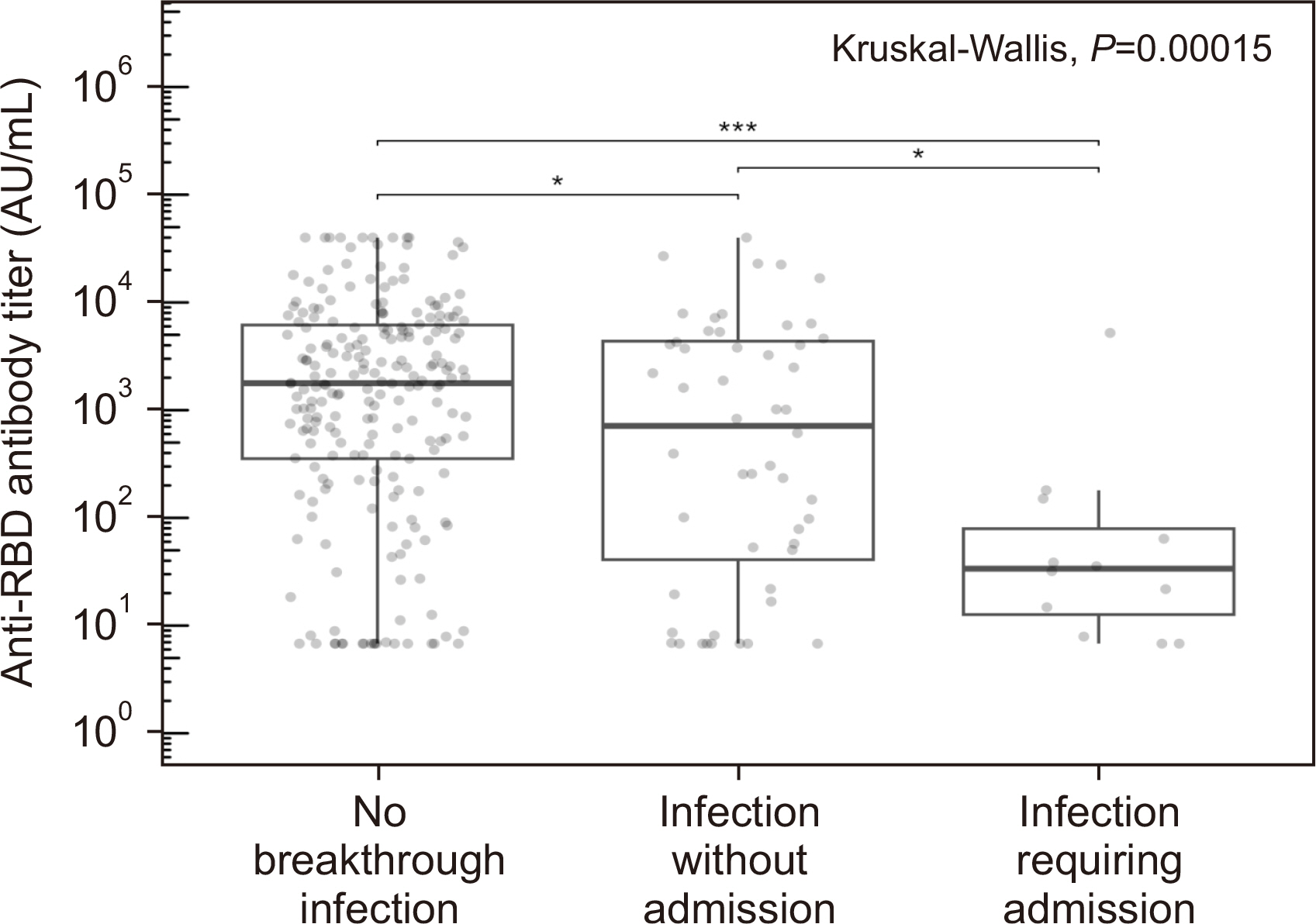
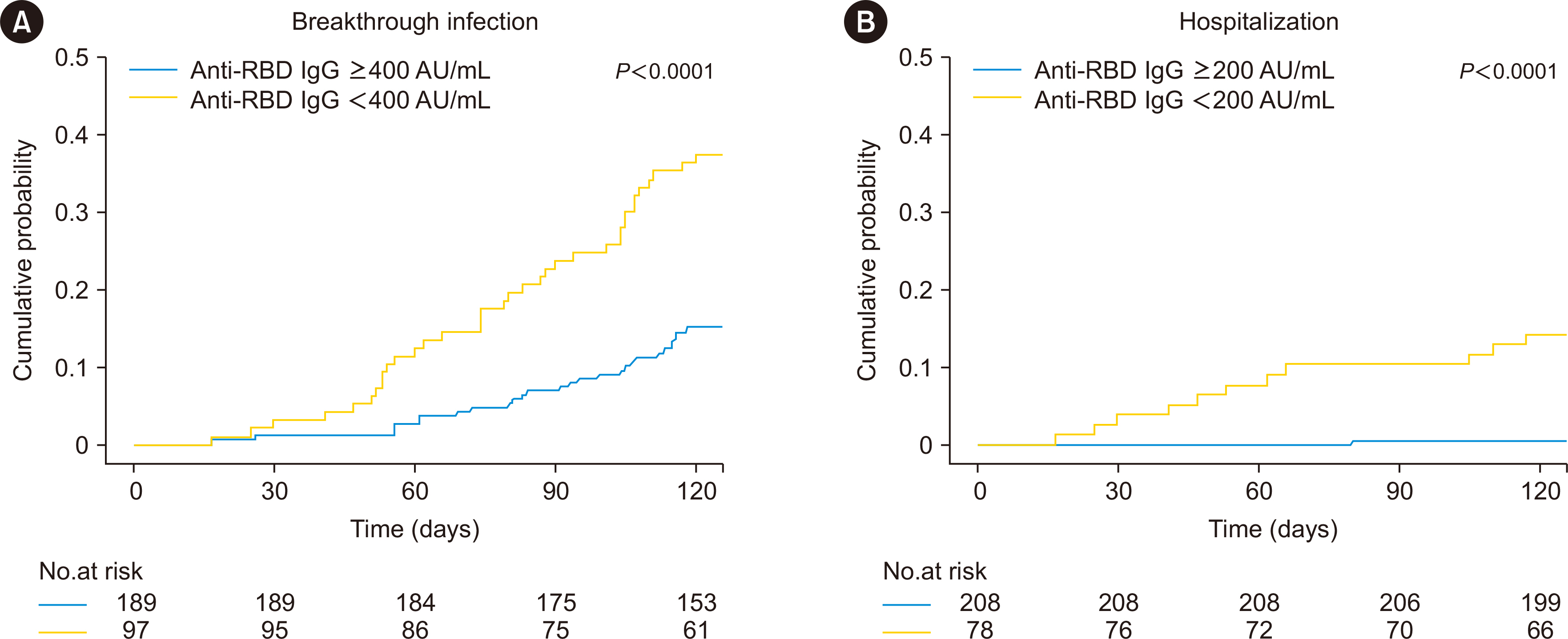
After the third vaccination, the seropositive rate and median antibody titer of the 287 patients increased from 57.1% to 82.2% and from 71.7 (interquartile range [IQR] 7.2– 402.8) to 1,612.1 (IQR 153.9–5,489.1) AU/mL, respectively. Sixty-four (22.3%) patients had symptomatic breakthrough infections, of whom 12 required hospitalization. Lower anti-receptor-binding domain (RBD) IgG levels ( < 400 AU/mL) post-third vaccination were a risk factor for symptomatic breakthrough infection (hazard ratio [HR] = 3.46, P < 0.001). Anti-RBD IgG levels < 200 AU/mL were a critical risk factor for hospitalization (HR = 36.4, P = 0.007).
Conclusions
Low anti-spike IgG levels after third vaccination in kidney transplant recipients were associated with symptomatic breakthrough infection and, particularly, with hospitalization during the Omicron surge. These data can be used to identify patients requiring additional protective measures, such as passive immunization using monoclonal antibodies.
Figure
Cited by 1 articles
-
New Insights Into SARS-CoV-2-specific Antibody Levels in Kidney Transplantation Recipients After Three Vaccination Doses
Hyunhye Kang, Eun-Jee Oh
Ann Lab Med. 2024;44(1):3-5. doi: 10.3343/alm.2024.44.1.3.
Reference
-
1. Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, et al. 2021; Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 385:e84. DOI: 10.1056/NEJMoa2114583. PMID: 34614326. PMCID: PMC8522797.
Article2. Coates PT, Wong G, Drueke T, Rovin B, Ronco P. Associate Editors, for the Entire Editorial Team. 2020; Early experience with COVID-19 in kidney transplantation. Kidney Int. 97:1074–5. DOI: 10.1016/j.kint.2020.04.001. PMID: 32354635. PMCID: PMC7142690.
Article3. Centers for Disease Control and Prevention. COVID-19: stay up to date with COVID-19 vaccines including boosters 2022. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/stay-up-to-date.html. Updated on Dec 2022.4. Benotmane I, Gautier G, Perrin P, Olagne J, Cognard N, Fafi-Kremer S, et al. 2021; Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA. 326:1063–5. DOI: 10.1001/jama.2021.12339. PMID: 34297036. PMCID: PMC8456389.
Article5. Cassaniti I, Gregorini M, Bergami F, Arena F, Sammartino JC, Percivalle E, et al. 2022; Effect of a third dose of SARS-CoV-2 mRNA BNT162b2 vaccine on humoral and cellular responses and serum anti-HLA antibodies in kidney transplant recipients. Vaccines (Basel). 10:921. DOI: 10.3390/vaccines10060921. PMID: 35746528. PMCID: PMC9227063.6. Lammert A, Schnuelle P, Rabenau HF, Ciesek S, Krämer BK, Göttmann U, et al. 2022; SARS-CoV-2 vaccination in kidney transplant recipients-stratified analysis of the humoral immune response. Transplant Direct. 8:e1384. DOI: 10.1097/TXD.0000000000001384. PMID: 36259077. PMCID: PMC9575732.
Article7. Massa F, Cremoni M, Gérard A, Grabsi H, Rogier L, Blois M, et al. 2021; Safety and cross-variant immunogenicity of a three-dose COVID-19 mRNA vaccine regimen in kidney transplant recipients. EBioMedicine. 73:103679. DOI: 10.1016/j.ebiom.2021.103679. PMID: 34763205. PMCID: PMC8573385.
Article8. Stumpf J, Tonnus W, Paliege A, Rettig R, Steglich A, Gembardt F, et al. 2021; Cellular and humoral immune responses after 3 doses of BNT162b2 mRNA SARS-CoV-2 vaccine in kidney transplant. Transplantation. 105:e267–9. DOI: 10.1097/TP.0000000000003903. PMID: 34342963. PMCID: PMC8549130.
Article9. Tylicki L, Dębska-Ślizień A, Muchlado M, Ślizień Z, Gołębiewska J, Dąbrowska M, et al. 2021; Boosting humoral immunity from mRNA COVID-19 vaccines in kidney transplant recipients. Vaccines (Basel). 10:56. DOI: 10.3390/vaccines10010056. PMID: 35062717. PMCID: PMC8779302.
Article10. Werbel WA, Boyarsky BJ, Ou MT, Massie AB, Tobian AAR, Garonzik-Wang JM, et al. 2021; Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med. 174:1330–2. DOI: 10.7326/L21-0282. PMID: 34125572. PMCID: PMC8252023.
Article11. Kim J, Bang HI, Shin JW, Park Y, Kim S, Kim M, et al. 2022; Immunogenicity of Third-dose BNT162b2 mRNA Vaccine Following Two Doses of ChAdOx1 in Health Care Workers: A Prospective Longitudinal Study. Ann Lab Med. 42:688–92. DOI: 10.3343/alm.2022.42.6.688. PMID: 35765878. PMCID: PMC9277035.
Article12. Altarawneh HN, Chemaitelly H, Hasan MR, Ayoub HH, Qassim S, AlMukdad S, et al. 2022; Protection against the Omicron variant from previous SARS-CoV-2 infection. N Engl J Med. 386:1288–90. DOI: 10.1056/NEJMc2200133. PMID: 35139269. PMCID: PMC8849180.
Article13. Chaguza C, Coppi A, Earnest R, Ferguson D, Kerantzas N, Warner F, et al. 2022; Rapid emergence of SARS-CoV-2 Omicron variant is associated with an infection advantage over Delta in vaccinated persons. Med. 3:325–34.e4. DOI: 10.1016/j.medj.2022.03.010. PMID: 35399324. PMCID: PMC8983481.
Article14. Tseng HF, Ackerson BK, Luo Y, Sy LS, Talarico CA, Tian Y, et al. 2022; Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat Med. 28:1063–71. DOI: 10.1038/s41591-022-01753-y. PMID: 35189624. PMCID: PMC9117141.
Article15. Werbel WA, Segev DL. 2022; SARS-CoV-2 antibody testing for transplant recipients: a tool to personalize protection versus COVID-19. Am J Transplant. 22:1316–20. DOI: 10.1111/ajt.16993. PMID: 35119179. PMCID: PMC9111420.16. Alejo JL, Chiang TPY, Bowles Zeiser L, Kim JD, Mitchell J, Avery RK, et al. 2022; Incidence and severity of COVID-19 among vaccinated solid organ transplant recipients during the Omicron wave. Transplantation. 106:e413–5. DOI: 10.1097/TP.0000000000004226. PMID: 35655363.17. Kemlin D, Gemander N, Depickère S, Olislagers V, Georges D, Waegemans A, et al. 2023; Humoral and cellular immune correlates of protection against COVID-19 in kidney transplant recipients. Am J Transplant. 23:649–58. DOI: 10.1016/j.ajt.2023.02.015. PMID: 36773936. PMCID: PMC9911984.
Article18. Bertrand D, Laurent C, Lemée V, Lebourg L, Hanoy M, Le Roy F, et al. 2022; Efficacy of anti-SARS-CoV-2 monoclonal antibody prophylaxis and vaccination on the Omicron variant of COVID-19 in kidney transplant recipients. Kidney Int. 102:440–2. DOI: 10.1016/j.kint.2022.05.007. PMID: 35618097. PMCID: PMC9125992.
Article19. Bonazzetti C, Tazza B, Gibertoni D, Pasquini Z, Caroccia N, Fanì F, et al. Relationship between immune response to severe acute respiratory syndrome coronavirus 2 vaccines and development of breakthrough infection in solid organ transplant recipients: the CONTRAST cohort. Clin Infect Dis. 2023; 76:1761–7. DOI: 10.1093/cid/ciad016. PMID: 36636955. PMCID: PMC10209432.
Article20. Hong KH, Kim GJ, Roh KH, Sung H, Lee J, Kim SY, et al. 2022; Update of guidelines for laboratory diagnosis of COVID-19 in Korea. Ann Lab Med. 42:391–7. DOI: 10.3343/alm.2022.42.4.391. PMID: 35177559. PMCID: PMC8859556.
Article21. Manothummetha K, Chuleerarux N, Sanguankeo A, Kates OS, Hirankarn N, Thongkam A, et al. 2022; Immunogenicity and risk factors associated with poor humoral immune response of SARS-CoV-2 vaccines in recipients of solid organ transplant: a systematic review and meta-analysis. JAMA Netw Open. 5:e226822. DOI: 10.1001/jamanetworkopen.2022.6822. PMID: 35412626. PMCID: PMC9006106.22. Rodríguez-Cubillo B, Moreno de la Higuera MA, Pérez-Flores I, Calvo Romero N, Aiffil AS, Arribi Vilela A, et al. 2022; Clinical effectiveness of SARS-CoV-2 vaccination in renal transplant recipients. Antibody levels impact in pneumonia and death. Transplantation. 106:e476–87. DOI: 10.1097/TP.0000000000004261. PMID: 35859270.
Article23. Yahav D, Rozen-Zvi B, Mashraki T, Atamna A, Ben-Zvi H, Bar-Haim E, et al. 2021; Immunosuppression reduction when administering a booster dose of the BNT162b2 mRNA SARS-CoV-2 vaccine in kidney transplant recipients without adequate humoral response following two vaccine doses: protocol for a randomised controlled trial (BECAME study). BMJ Open. 11:e055611. DOI: 10.1136/bmjopen-2021-055611. PMID: 34635537. PMCID: PMC8506046.
Article24. Kumari M, Lu RM, Li MC, Huang JL, Hsu FF, Ko SH, et al. 2022; A critical overview of current progress for COVID-19: development of vaccines, antiviral drugs, and therapeutic antibodies. J Biomed Sci. 29:68. DOI: 10.1186/s12929-022-00852-9. PMID: 36096815. PMCID: PMC9465653.25. Al Jurdi A, Morena L, Cote M, Bethea E, Azzi J, Riella LV. 2022; Tixagevimab/cilgavimab pre-exposure prophylaxis is associated with lower breakthrough infection risk in vaccinated solid organ transplant recipients during the Omicron wave. Am J Transplant. 22:3130–6. DOI: 10.1111/ajt.17128. PMID: 35727916. PMCID: PMC9906353.
Article26. Avery RK. 2022; Update on COVID-19 therapeutics for solid organ transplant recipients, including the Omicron surge. Transplantation. 106:1528–37. DOI: 10.1097/TP.0000000000004200. PMID: 35700481. PMCID: PMC9311293.
Article27. Li J, Ayada I, Wang Y, den Hoed CM, Kamar N, Peppelenbosch MP, et al. 2022; Factors associated with COVID-19 vaccine response in transplant recipients: a systematic review and meta-analysis. Transplantation. 106:2068–75. DOI: 10.1097/TP.0000000000004256. PMID: 35761439. PMCID: PMC9521391.
Article28. Kho MML, Messchendorp AL, Frölke SC, Imhof C, Koomen VJ, Malahe SRK, et al. 2023; Alternative strategies to increase the immunogenicity of COVID-19 vaccines in kidney transplant recipients not responding to two or three doses of an mRNA vaccine (RECOVAC): a randomised clinical trial. Lancet Infect Dis. 23:307–19. DOI: 10.1016/S1473-3099(22)00650-8. PMID: 36354032.
Article29. Kühn T, Speer C, Morath C, Bartenschlager M, Kim H, Beimler J, et al. 2023; Immune response to COVID-19 mRNA vaccination in previous nonresponder kidney transplant recipients after short-term withdrawal of mycophenolic acid 1 and 3 months after an additional vaccine dose. Transplantation. 107:1139–50. DOI: 10.1097/TP.0000000000004516. PMID: 36617671. PMCID: PMC10125015.
Article30. Benotmane I, Velay A, Gautier-Vargas G, Olagne J, Obrecht A, Cognard N, et al. 2022; Breakthrough COVID-19 cases despite prophylaxis with 150 mg of tixagevimab and 150 mg of cilgavimab in kidney transplant recipients. Am J Transplant. 22:2675–81. DOI: 10.1111/ajt.17121. PMID: 35713984. PMCID: PMC9350296.
Article31. Karaba AH, Kim JD, Chiang TP, Alejo JL, Sitaras I, Abedon AT, et al. 2023; Neutralizing activity and 3-month durability of tixagevimab and cilgavimab prophylaxis against Omicron sublineages in transplant recipients. Am J Transplant. 23:423–8. DOI: 10.1016/j.ajt.2022.11.002. PMID: 36906295. PMCID: PMC9835002.
Article32. Gilbert PB, Montefiori DC, McDermott AB, Fong Y, Benkeser D, Deng W, et al. 2022; Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 375:43–50. DOI: 10.1126/science.abm3425. PMID: 34812653. PMCID: PMC9017870.
Article33. Simon G, Favresse J, Gillot C, Closset M, Catry É, Dogné JM, et al. Kinetics and ability of binding antibody and surrogate virus neutralization tests to predict neutralizing antibodies against the SARS-CoV-2 Omicron variant following BNT162b2 booster administration. Clin Chem Lab Med. 2023; DOI: 10.1515/cclm-2022-1258. PMID: 37078220.
Article34. Suntronwong N, Assawakosri S, Kanokudom S, Yorsaeng R, Auphimai C, Thongmee T, et al. 2022; Strong correlations between the binding antibodies against wild-type and neutralizing antibodies against Omicron BA.1 and BA.2 variants of SARS-CoV-2 in individuals following booster (third-dose) vaccination. Diagnostics (Basel). 12:1781. DOI: 10.3390/diagnostics12081781. PMID: 35892491. PMCID: PMC9394243.
Article35. Le Bert N, Chia WN, Wan WY, Teo AKJ, Chong SZ-R, Tan N, et al. 2021; Widely heterogeneous humoral and cellular immunity after mild SARS-CoV-2 infection in a homogeneous population of healthy young men. Emerg Microbes Infect. 10:2141–50. DOI: 10.1080/22221751.2021.1999777. PMID: 34709140. PMCID: PMC8604544.
Article36. Malipiero G, Moratto A, Infantino M, D'Agaro P, Piscianz E, Manfredi M, et al. 2021; Assessment of humoral and cellular immunity induced by the BNT162b2 SARS-CoV-2 vaccine in healthcare workers, elderly people, and immunosuppressed patients with autoimmune disease. Immunol Res. 69:576–83. DOI: 10.1007/s12026-021-09226-z. PMID: 34417958. PMCID: PMC8379062.
Article37. Seo YJ, Oh I, Nam M, Shin S, Roh EY, Song EY. 2023; Comparison of Four T-cell Assays and Two Binding Antibody Assays in SARS-CoV-2 Vaccinees With or Without Omicron Breakthrough Infection. Ann Lab Med. 43:596–604. DOI: 10.3343/alm.2023.43.6.596. PMID: 37387492. PMCID: PMC10345181.
Article38. Kuhlmann C, Mayer CK, Claassen M, Maponga T, Burgers WA, Keeton R, et al. 2022; Breakthrough infections with SARS-CoV-2 omicron despite mRNA vaccine booster dose. Lancet. 399:625–6. DOI: 10.1016/S0140-6736(22)00090-3. PMID: 35063123.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-SARS-CoV-2 spike antibody response to the third dose of BNT162b2 mRNA COVID-19 vaccine and associated factors in Japanese hemodialysis patients
- Immunogenicity of SARS-CoV-2 vaccine in kidney transplant recipients: a cross-sectional study in Korea
- Anti-spike antibody titer in kidney transplant recipients after the third dose of SARS-CoV-2 vaccination correlates with the incidence and severity of breakthrough infection during the Omicron surge
- New Insights Into SARS-CoV-2-specific Antibody Levels in Kidney Transplantation Recipients After Three Vaccination Doses
- Immunogenicity of SARS-CoV-2 Vaccine in Kidney Transplant Recipients: A Cross-Sectional Study in Korea