Endocrinol Metab.
2023 Dec;38(6):720-729. 10.3803/EnM.2023.1758.
Comparative Analysis of Driver Mutations and Transcriptomes in Papillary Thyroid Cancer by Region of Residence in South Korea
- Affiliations
-
- 1Department of Surgery, Open NBI Convergence Technology Research Laboratory, Severance Hospital, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea
- 2Division of Endocrinology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
- 3Radiation Effect Research Section, Radiation Health Institute, Korea Hydro & Nuclear Power Co., Ltd., Gyeongju, Korea
- KMID: 2549265
- DOI: http://doi.org/10.3803/EnM.2023.1758
Abstract
- Background
Radiation exposure is a well-known risk factor for papillary thyroid cancer (PTC). South Korea has 24 nuclear reactors in operation; however, no molecular biological analysis has been performed on patients with PTC living near nuclear power plants.
Methods
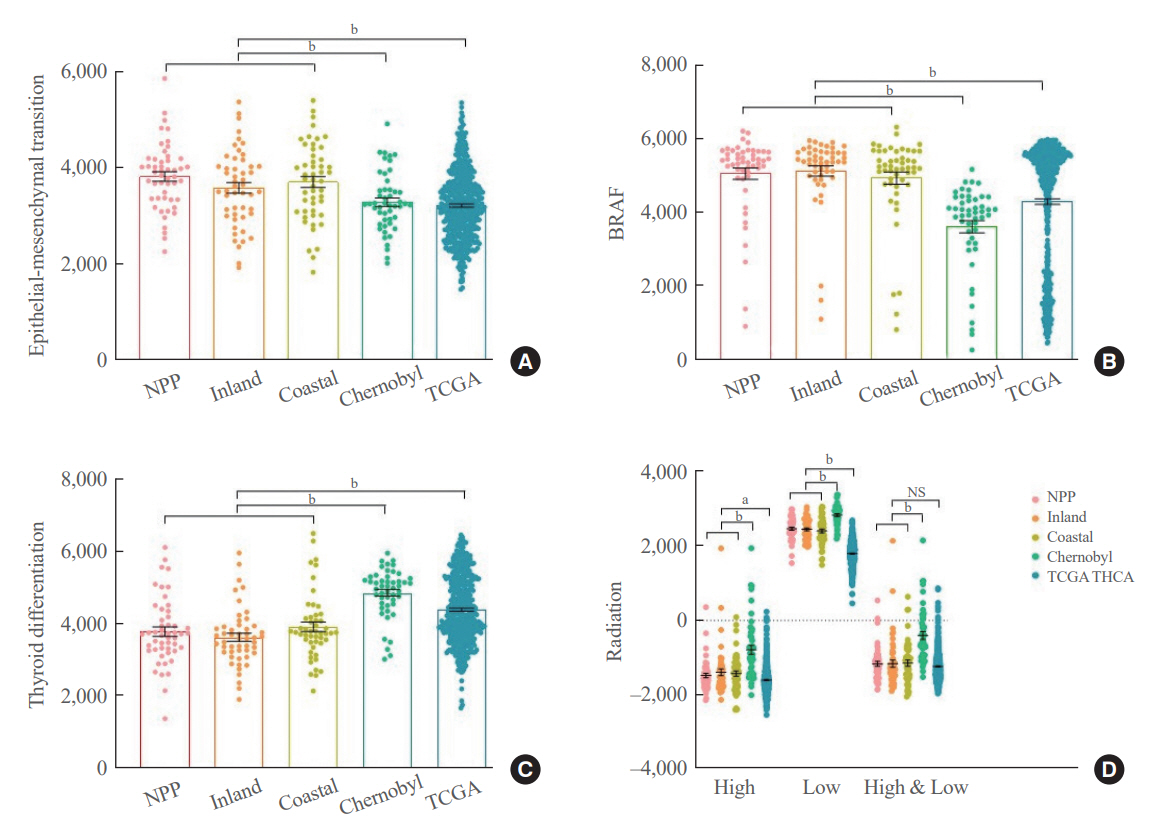
We retrospectively included patients with PTC (n=512) divided into three groups according to their place of residence at the time of operation: inland areas (n=300), coastal areas far from nuclear power plants (n=134), and nuclear power plant areas (n=78). After propensity score matching (1:1:1) by age, sex, and surgical procedure, the frequency of representative driver mutations and gene expression profiles were compared (n=50 per group). Epithelial-mesenchymal transition (EMT), BRAF, thyroid differentiation, and radiation scores were calculated and compared.
Results
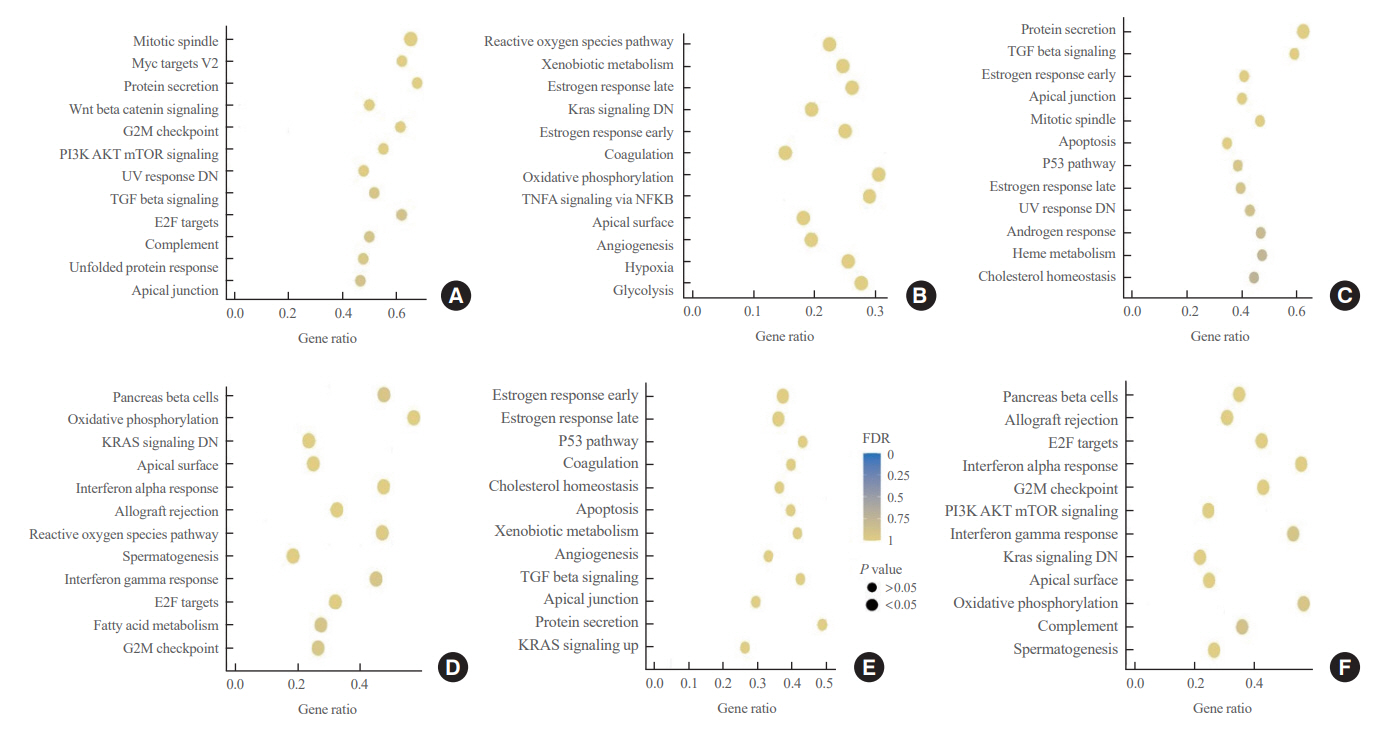
No significant difference was observed in clinicopathological characteristics, including radiation exposure history and the frequency of incidentally discovered thyroid cancer, among the three groups. BRAFV600E mutation was most frequently detected in the groups, with no difference among the three groups. Furthermore, gene expression profiles showed no statistically significant difference. EMT and BRAF scores were higher in our cohort than in cohorts from Chernobyl tissue bank and The Cancer Genome Atlas Thyroid Cancer; however, there was no difference according to the place of residence. Radiation scores were highest in the Chernobyl tissue bank but exhibited no difference according to the place of residence.
Conclusion
Differences in clinicopathological characteristics, frequency of representative driver mutations, and gene expression profiles were not observed according to patients’ region of residence in South Korea.
Keyword
Figure
Reference
-
1. Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988-2005. Cancer. 2009; 115:3801–7.
Article2. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133.
Article3. Oh CM, Won YJ, Jung KW, Kong HJ, Cho H, Lee JK, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2013. Cancer Res Treat. 2016; 48:436–50.
Article4. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009; 19:1167–214.
Article5. Hughes DT, Haymart MR, Miller BS, Gauger PG, Doherty GM. The most commonly occurring papillary thyroid cancer in the United States is now a microcarcinoma in a patient older than 45 years. Thyroid. 2011; 21:231–6.
Article6. Ito Y, Miyauchi A, Oda H. Low-risk papillary microcarcinoma of the thyroid: a review of active surveillance trials. Eur J Surg Oncol. 2018; 44:307–15.
Article7. Shakhtarin VV, Tsyb AF, Stepanenko VF, Orlov MY, Kopecky KJ, Davis S. Iodine deficiency, radiation dose, and the risk of thyroid cancer among children and adolescents in the Bryansk region of Russia following the Chernobyl power station accident. Int J Epidemiol. 2003; 32:584–91.
Article8. Kitahara CM, Platz EA, Freeman LE, Hsing AW, Linet MS, Park Y, et al. Obesity and thyroid cancer risk among U.S. men and women: a pooled analysis of five prospective studies. Cancer Epidemiol Biomarkers Prev. 2011; 20:464–72.
Article9. Lee J, Lee CR, Ku CR, Kang SW, Jeong JJ, Shin DY, et al. Association between obesity and BRAFV600E mutation status in patients with papillary thyroid cancer. Ann Surg Oncol. 2015; 22 Suppl 3:S683–90.10. Son H, Lee H, Kang K, Lee I. The risk of thyroid cancer and obesity: a nationwide population-based study using the Korea National Health Insurance Corporation cohort database. Surg Oncol. 2018; 27:166–71.
Article11. Stone TW, McPherson M, Gail Darlington L. Obesity and cancer: existing and new hypotheses for a causal connection. EBioMedicine. 2018; 30:14–28.
Article12. Yamamoto H, Hayashi K, Scherb H. Association between the detection rate of thyroid cancer and the external radiation dose-rate after the nuclear power plant accidents in Fukushima, Japan. Medicine (Baltimore). 2019; 98:e17165.
Article13. Zidane M, Cazier JB, Chevillard S, Ory C, Schlumberger M, Dupuy C, et al. Genetic susceptibility to radiation-related differentiated thyroid cancers: a systematic review of literature. Endocr Relat Cancer. 2019; 26:R583–96.
Article14. Albi E, Cataldi S, Lazzarini A, Codini M, Beccari T, Ambesi-Impiombato FS, et al. Radiation and thyroid cancer. Int J Mol Sci. 2017; 18:911.
Article15. Furukawa K, Preston D, Funamoto S, Yonehara S, Ito M, Tokuoka S, et al. Long-term trend of thyroid cancer risk among Japanese atomic-bomb survivors: 60 years after exposure. Int J Cancer. 2013; 132:1222–6.
Article16. Nagataki S, Takamura N. Radioactive doses - predicted and actual - and likely health effects. Clin Oncol (R Coll Radiol). 2016; 28:245–54.
Article17. Rodgers BE, Holmes KM. Radio-adaptive response to environmental exposures at Chernobyl. Dose Response. 2008; 6:209–21.
Article18. Ivanov VK, Gorski AI, Maksioutov MA, Vlasov OK, Godko AM, Tsyb AF, et al. Thyroid cancer incidence among adolescents and adults in the Bryansk region of Russia following the Chernobyl accident. Health Phys. 2003; 84:46–60.
Article19. Kim JM, Kim MH, Ju YS, Hwang SS, Ha M, Kim BK, et al. Reanalysis of epidemiological investigation of cancer risk among people residing near nuclear power plants in South Korea. Int J Environ Res Public Health. 2018; 15:481.
Article20. Thomas GA, Bunnell H, Cook HA, Williams ED, Nerovnya A, Cherstvoy ED, et al. High prevalence of RET/PTC rearrangements in Ukrainian and Belarussian post-Chernobyl thyroid papillary carcinomas: a strong correlation between RET/PTC3 and the solid-follicular variant. J Clin Endocrinol Metab. 1999; 84:4232–8.
Article21. Morton LM, Karyadi DM, Stewart C, Bogdanova TI, Dawson ET, Steinberg MK, et al. Radiation-related genomic profile of papillary thyroid carcinoma after the Chernobyl accident. Science. 2021; 372:e. abg2538.
Article22. Nikiforov YE. RET/PTC rearrangement in thyroid tumors. Endocr Pathol. 2002; 13:3–16.
Article23. Jo YS, Li S, Song JH, Kwon KH, Lee JC, Rha SY, et al. Influence of the BRAF V600E mutation on expression of vascular endothelial growth factor in papillary thyroid cancer. J Clin Endocrinol Metab. 2006; 91:3667–70.
Article24. Guan H, Ji M, Bao R, Yu H, Wang Y, Hou P, et al. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab. 2009; 94:1612–7.
Article25. Kim HJ, Park HK, Byun DW, Suh K, Yoo MH, Min YK, et al. Iodine intake as a risk factor for BRAF mutations in papillary thyroid cancer patients from an iodine-replete area. Eur J Nutr. 2018; 57:809–15.
Article26. Mitsutake N, Fukushima T, Matsuse M, Rogounovitch T, Saenko V, Uchino S, et al. BRAF(V600E) mutation is highly prevalent in thyroid carcinomas in the young population in Fukushima: a different oncogenic profile from Chernobyl. Sci Rep. 2015; 5:16976.
Article27. Iwadate M, Mitsutake N, Matsuse M, Fukushima T, Suzuki S, Matsumoto Y, et al. The clinicopathological results of thyroid cancer with BRAFV600E mutation in the young population of Fukushima. J Clin Endocrinol Metab. 2020; 105:dgaa573.28. Seo DH, Lee SG, Lee HY, Jeong S, Park S, Lee J, et al. Lymph node metastasis-dependent molecular classification in papillary thyroid carcinoma defines aggressive metastatic outgrowth. Clin Transl Med. 2023; 13:e1211.
Article29. Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. 2013; 500:415–21.30. Groelly FJ, Fawkes M, Dagg RA, Blackford AN, Tarsounas M. Targeting DNA damage response pathways in cancer. Nat Rev Cancer. 2023; 23:78–94.
Article31. Cho IS, Song M, Choi Y, Li ZM, Ahn YO. Power estimation and follow-up period evaluation in Korea radiation effect and epidemiology cohort study. J Prev Med Public Health. 2010; 43:543–8.
Article32. Ahn YO, Li ZM; KREEC Study Group. Cancer risk in adult residents near nuclear power plants in Korea: a cohort study of 1992-2010. J Korean Med Sci. 2012; 27:999–1008.
Article33. Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014; 159:676–90.34. Song YS, Park YJ. Genomic characterization of differentiated thyroid carcinoma. Endocrinol Metab (Seoul). 2019; 34:1–10.
Article35. Wu S, Liu Y, Li K, Liang Z, Zeng X. Molecular and cytogenetic features of NTRK fusions enriched in BRAF and RET double-negative papillary thyroid cancer. J Mol Diagn. 2023; 25:569–82.
Article36. Wada N, Duh QY, Sugino K, Iwasaki H, Kameyama K, Mimura T, et al. Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann Surg. 2003; 237:399–407.37. Tuttle RM, Haugen B, Perrier ND. Updated American Joint Committee on Cancer/tumor-node-metastasis staging system for differentiated and anaplastic thyroid cancer (eighth edition): what changed and why? Thyroid. 2017; 27:751–6.
Article38. Martin SA, Dehler CE, Krol E. Transcriptomic responses in the fish intestine. Dev Comp Immunol. 2016; 64:103–17.
Article39. Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003; 34:267–73.
Article40. Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005; 102:15545–50.
Article41. Thomas GA, Bethel JA, Galpine A, Mathieson W, Krznaric M, Unger K. Integrating research on thyroid cancer after Chernobyl: the Chernobyl Tissue Bank. Clin Oncol (R Coll Radiol). 2011; 23:276–81.42. Lu TP, Hsu YY, Lai LC, Tsai MH, Chuang EY. Identification of gene expression biomarkers for predicting radiation exposure. Sci Rep. 2014; 4:6293.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Active Surveillance of Papillary Thyroid Cancer: Past, Present, and Future
- Effect of Afatinib for Lung Cancer on Papillary Thyroid Carcinoma
- A Case of Ectopic Thyroid Papillary Carcinoma with Incidental Papillary Thyroid Microcarcinoma
- Incidence and clinical characteristics of thyroid cancer in Korea
- Thyroid Lobectomy as an Initial Treatment Option on 1-4 cm Papillary Cancer