Restor Dent Endod.
2022 Nov;47(4):e42. 10.5395/rde.2022.47.e42.
The influence of sodium hypochlorite concentration on the fibrin structure of human blood clots and transforming growth factor-beta 1 release: an ex vivo study
- Affiliations
-
- 1Department of Conservative Dentistry and Endodontics, Faculty of Dentistry, Meenakshi Ammal Dental College & Hospital, Meenakshi Academy of Higher Education and Research (MAHER), Chennai, TN, India
- KMID: 2548146
- DOI: http://doi.org/10.5395/rde.2022.47.e42
Abstract
Objective
This study investigated the effects of various concentrations of sodium hypochlorite (NaOCl) on human whole-blood clotting kinetics, the structure of the blood clots formed, and transforming growth factor (TGF)-β1 release.
Materials and Methods
Human whole blood was collected from 5 healthy volunteers and divided into 4 groups: CG (control, 0.5 mL of blood), BN0.5 (0.5 mL of blood with 0.5 mL of 0.5% NaOCl), BN3 (0.5 mL of blood with 0.5 mL of 3% NaOCl), and BN5.25 (0.5 mL of blood with 0.5 mL of 5.25% NaOCl). The effects of NaOCl on clotting kinetics, structure of fibrin and cells, and release of TGF-β1 were assessed using thromboelastography (TEG), scanning electron microscopy (SEM), and enzyme-linked immunosobent assay, respectively. Statistical analysis was conducted using the Kruskal Wallis and Mann-Whitney U tests, followed by the post hoc Dunn test. A p value < 0.05 indicated statistical significance.
Results
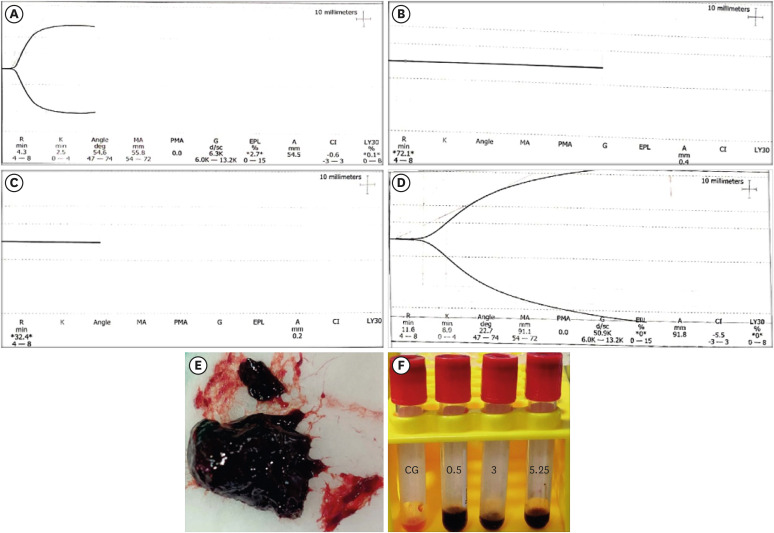
The blood samples in BN0.5 and BN3 did not clot, whereas the TEG of BN5.25 showed altered clot formation. Samples from the CG and BN 3 groups could only be processed with SEM, which showed that the latter lacked fibrin formation and branching of fibers, as well as clumping of red blood cells with surface roughening and distortion. TGF-β1 release was significantly highest in BN 3 when all groups were compared to CG (p < 0.05).
Conclusions
Each concentration of NaOCl affected the release of TGF-β1 from blood clots and altered the clotting mechanism of blood by affecting clotting kinetics and cell structure.
Keyword
Figure
Reference
-
1. Monroe DM, Hoffman M. The clotting system - a major player in wound healing. Haemophilia. 2012; 18(Supplement 5):11–16. PMID: 22757679.
Article2. Laurens N, Koolwijk P, de Maat MP. Fibrin structure and wound healing. J Thromb Haemost. 2006; 4:932–939. PMID: 16689737.
Article3. Saber SE. Tissue engineering in endodontics. J Oral Sci. 2009; 51:495–507. PMID: 20032600.
Article4. American Association of Endodontists. Glossary of endodontic terms. 10th ed. Chicago, IL: American Association of Endodontists;2020.5. European Society of Endodontology (ESE). Duncan HF, Galler KM, Tomson PL, Simon S, El-Karim I, Kundzina R, Krastl G, Dammaschke T, Fransson H, Markvart M, Zehnder M, Bjørndal L. European Society of Endodontology position statement: Management of deep caries and the exposed pulp. Int Endod J. 2019; 52:923–934. PMID: 30664240.
Article6. Sugiyama S, Kugiyama K, Aikawa M, Nakamura S, Ogawa H, Libby P. Hypochlorous acid, a macrophage product, induces endothelial apoptosis and tissue factor expression: involvement of myeloperoxidase-mediated oxidant in plaque erosion and thrombogenesis. Arterioscler Thromb Vasc Biol. 2004; 24:1309–1314. PMID: 15142860.
Article7. Di Stasio E, Nagaswami C, Weisel JW, Di Cera E. Cl- regulates the structure of the fibrin clot. Biophys J. 1998; 75:1973–1979. PMID: 9746538.
Article8. Standeven KF, Ariëns RA, Grant PJ. The molecular physiology and pathology of fibrin structure/function. Blood Rev. 2005; 19:275–288. PMID: 15963835.
Article9. Moorer WR, Wesselink PR. Factors promoting the tissue dissolving capability of sodium hypochlorite. Int Endod J. 1982; 15:187–196. PMID: 6964523.
Article10. Pashley EL, Birdsong NL, Bowman K, Pashley DH. Cytotoxic effects of NaOCl on vital tissue. J Endod. 1985; 11:525–528. PMID: 3867719.
Article11. Yamaguchi H, Hosoya N, Kobayashi K, Yokota T, Arai T, Nakamura J, Cox CF. The influence of two concentrations of sodium hypochlorite on human blood: changes in haemolysis, pH and protein. Int Endod J. 2001; 34:231–236. PMID: 12193269.
Article12. Nagendrababu V, Murray PE, Ordinola-Zapata R, Peters OA, Rôças IN, Siqueira JF Jr, Priya E, Jayaraman J, Pulikkotil SJ, Suresh N, Dummer PM. PRILE 2021 guidelines for reporting laboratory studies in Endodontology: explanation and elaboration. Int Endod J. 2021; 54:1491–1515. PMID: 33982298.
Article13. World Health Organization. WHO guidelines on drawing blood: best practices in phlebotomy. Geneva: World Health Organization;2010.14. Dean AG, Sullivan KM, Soe MM. OpenEpi: open source epidemiologic statistics for public health, version. updated April 6, 2013. 2022. Available from: www.OpenEpi.com.15. Chernysh IN, Weisel JW. Dynamic imaging of fibrin network formation correlated with other measures of polymerization. Blood. 2008; 111:4854–4861. PMID: 18272815.
Article16. Shaydakov ME, Sigmon DF, Blebea J. Thromboelastography [Internet]. Treasure Island, FL: StatPearls Publishing;2022.17. da Rosa WL, Piva E, da Silva AF. Disclosing the physiology of pulp tissue for vital pulp therapy. Int Endod J. 2018; 51:829–846. PMID: 29405371.
Article18. Štikarová J, Kotlín R, Riedel T, Suttnar J, Pimková K, Chrastinová L, Dyr JE. The effect of reagents mimicking oxidative stress on fibrinogen function. Sci World J. 2013; 2013:359621.
Article19. Murina MA, Roshchupkin DI, Kravchenko NN, Petrova AO, Sergienko VI. Antiaggregant effects of biogenic chloramines. Bull Exp Biol Med. 2007; 144:464–470. PMID: 18457056.
Article20. Azizova OA, Aseichev AV, Piryazev AP, Roitman EV, Shcheglovitova ON. Effects of oxidized fibrinogen on the functions of blood cells, blood clotting, and rheology. Bull Exp Biol Med. 2007; 144:397–407. PMID: 18457045.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expressions of transforming growth factor beta in patients with rheumatioid arthritis and osteoarthritis
- Transforming growth factor-beta promoted vascular endothelial growth factor release by human lung fibroblasts
- Precipitate from a combination of sodium hypochlorite and chlorhexidine
- Transforming growth factor-beta 1 responsiveness of human articular chondrocytes in vitro: normal versus osteoarthritis
- The Effect of Transforming Growth Factor-beta and Mannose-6-Phosphate on the Proliferation of Subconjunctival Fibroblast of Rabbit