Restor Dent Endod.
2022 Feb;47(1):e12. 10.5395/rde.2022.47.e12.
Interplay of collagen and mast cells in periapical granulomas and periapical cysts: a comparative polarizing microscopic and immunohistochemical study
- Affiliations
-
- 1Department of Oral and Maxillofacial Pathology and Microbiology, Post Graduate Institute of Dental Sciences, Pt B.D. Sharma University of Health Sciences, Rohtak, India
- 2Department of Pathology, Post Graduate Institute of Medical Sciences, Pt B.D. Sharma University of Health Sciences, Rohtak, India
- KMID: 2548107
- DOI: http://doi.org/10.5395/rde.2022.47.e12
Abstract
Objectives
This pilot study aimed to establish the interrelationship between collagen and mast cells in periapical granulomas and periapical cysts.
Materials and Methods
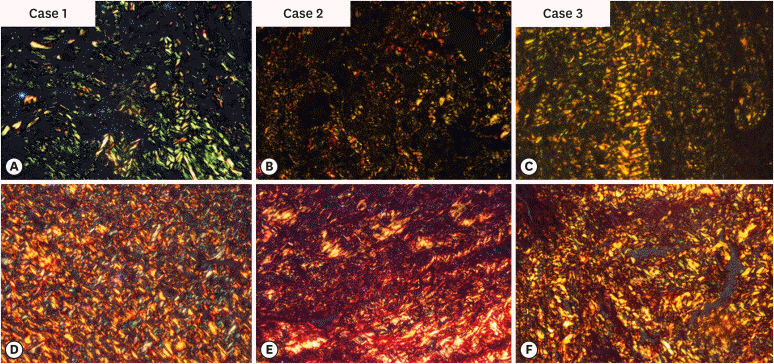
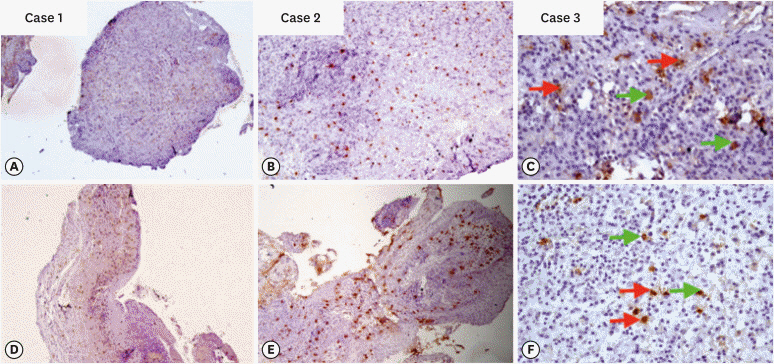
An observational cross-sectional study was conducted on the paraffin-embedded tissue sections of 68 specimens (34 periapical granulomas and 34 periapical cysts). The specimens were stained with picrosirius to observe collagen fiber birefringence and anti-tryptase antibody to evaluate the mast cell count immunohistochemically. The mean number and birefringence of collagen fibers, as well as the mean number of mast cells (total, granulated, and degranulated), and the mean inflammatory cell density were calculated. The data obtained were analyzed using the Kruskal Wallis test, Mann Whitney U test, and Spearman correlation test (p < 0.05).
Results
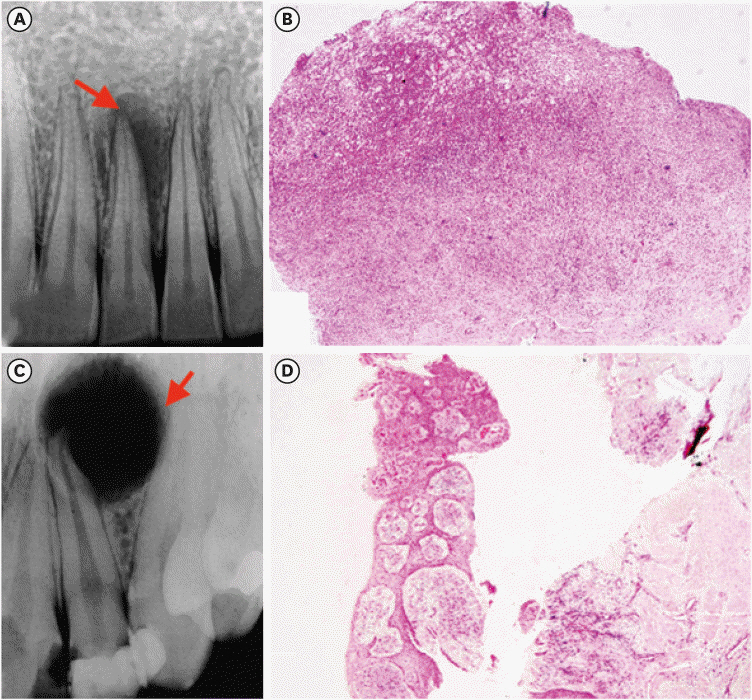
The mean number of thick collagen fibers was higher in periapical cysts, while that of thin fibers was higher in granulomas (p = 0.00). Cysts emitted orange-yellow to red birefringence, whereas periapical granulomas had predominantly green fibers (p = 0.00). The mean inflammatory cell density was comparable in all groups (p = 0.129). The number of total, degranulated, and granulated mast cells exhibited significant results (p = 0.00) in both groups. Thick cyst fibers showed significant inverse correlations with inflammation and degranulated mast cells (p = 0.041, 0.04 respectively).
Conclusions
Mast cells and inflammatory cells influenced the nature of collagen fiber formation and its birefringence. This finding may assist in the prediction of the nature, pathogenesis, and biological behavior of periapical lesions.
Figure
Reference
-
1. Vernal R, Dezerega A, Dutzan N, Chaparro A, León R, Chandía S, Silva A, Gamonal J. RANKL in human periapical granuloma: possible involvement in periapical bone destruction. Oral Dis. 2006; 12:283–289. PMID: 16700737.
Article2. Ledesma-Montes C, Garcés-Ortíz M, Rosales-García G, Hernández-Guerrero JC. Importance of mast cells in human periapical inflammatory lesions. J Endod. 2004; 30:855–859. PMID: 15564863.
Article3. Marçal JR, Samuel RO, Fernandes D, de Araujo MS, Napimoga MH, Pereira SA, Clemente-Napimoga JT, Alves PM, Mattar R, Rodrigues V Jr, Rodrigues DB. T-helper cell type 17/regulatory T-cell immunoregulatory balance in human radicular cysts and periapical granulomas. J Endod. 2010; 36:995–999. PMID: 20478453.
Article4. Syed Ismail PM, Apoorva K, Manasa N, Rama Krishna R, Bhowmick S, Jain S. Clinical, radiographic, and histological findings of chronic inflammatory periapical lesions: a clinical study. J Family Med Prim Care. 2020; 9:235–238. PMID: 32110596.
Article5. Natanasabapathy V, Arul B, Mishra A, Varghese A, Padmanaban S, Elango S, Arockiam S. Ultrasound imaging for the differential diagnosis of periapical lesions of endodontic origin in comparison with histopathology: a systematic review and meta-analysis. Int Endod J. 2021; 54:693–711. PMID: 33368404.
Article6. Juerchott A, Pfefferle T, Flechtenmacher C, Mente J, Bendszus M, Heiland S, Hilgenfeld T. Differentiation of periapical granulomas and cysts by using dental MRI: a pilot study. Int J Oral Sci. 2018; 10:17. PMID: 29777107.
Article7. Ji HJ, Park SH, Cho KM, Lee SK, Kim JW. Differential diagnosis of periapical cyst using collagen birefringence pattern of the cyst wall. Restor Dent Endod. 2017; 42:111–117. PMID: 28503476.
Article8. Jahagirdar PB, Kale AD, Hallikerimath S. Stromal characterization and comparison of odontogenic cysts and odontogenic tumors using picrosirius red stain and polarizing microscopy: A retrospective and histochemical study. Indian J Cancer. 2015; 52:408–412. PMID: 26905155.
Article9. El Safoury OS, Fawzy MM, El Maadawa ZM, Mohamed DH. Quantitation of mast cells and collagen fibers in skin tags. Indian J Dermatol. 2009; 54:319–322. PMID: 20101330.
Article10. Vij R, Vij H, Rao NN. Evaluation of collagen in connective tissue walls of odontogenic cysts--a histochemical study. J Oral Pathol Med. 2011; 40:257–262. PMID: 20969631.
Article11. França GM, Carmo AF, Costa Neto H, Andrade AL, Lima KC, Galvão HC. Macrophages subpopulations in chronic periapical lesions according to clinical and morphological aspects. Braz Oral Res. 2019; 33:e047. PMID: 31141038.
Article12. Yong LC. The mast cell: origin, morphology, distribution, and function. Exp Toxicol Pathol. 1997; 49:409–424. PMID: 9495641.
Article13. Costa Neto H, de Andrade AL, Gordón-Núñez MA, Freitas RA, Galvão HC. Immunoexpression of tryptase-positive mast cells in periapical granulomas and radicular cysts. Int Endod J. 2015; 48:729–735. PMID: 25100244.
Article14. Andrade AL, Santos EM, Carmo AF, Freitas RA, Galvão HC. Analysis of tryptase-positive mast cells and immunoexpression of MMP-9 and MMP-13 in periapical lesions. Int Endod J. 2017; 50:446–454. PMID: 27003572.
Article15. Floratos S, Kim S. Modern Endodontic Microsurgery Concepts: A Clinical Update. Dent Clin North Am. 2017; 61:81–91. PMID: 27912820.16. Ankle MR, Joshi PS. A study to evaluate the efficacy of xylene-free hematoxylin and eosin staining procedure as compared to the conventional hematoxylin and eosin staining: an experimental study. J Oral Maxillofac Pathol. 2011; 15:161–167. PMID: 22529574.
Article17. Hirshberg A, Lib M, Kozlovsky A, Kaplan I. The influence of inflammation on the polarization colors of collagen fibers in the wall of odontogenic keratocyst. Oral Oncol. 2007; 43:278–282. PMID: 16919995.
Article18. Puchtler H, Waldrop FS, Valentine LS. Polarization microscopic studies of connective tissue stained with picro-sirius red FBA. Beitr Pathol. 1973; 150:174–187. PMID: 4129194.
Article19. Hirshberg A, Sherman S, Buchner A, Dayan D. Collagen fibres in the wall of odontogenic keratocysts: a study with picrosirius red and polarizing microscopy. J Oral Pathol Med. 1999; 28:410–412. PMID: 10535364.
Article20. Shetty A, Tamgadge A, Bhalerao S, et al. Study of polarization colors in the connective tissue wall of odontogenic cysts using picrosirius red stain. J Orofac Sci. 2015; 7:119–124.
Article21. Singh HP, Shetty DC, Wadhwan V, Aggarwal P. A quantitative and qualitative comparative analysis of collagen fibers to determine the role of connective tissue stroma on biological behavior of odontogenic cysts: a histochemical study. Natl J Maxillofac Surg. 2012; 3:15–20. PMID: 23251052.
Article22. Mahajan AM, Mahajan MC, Ganvir SM, Hazarey VK. The role of stroma in the expansion of odontogenic cysts and adenomatoid odontogenic tumor: a polarized microscopy study. J Nat Sci Biol Med. 2013; 4:316–320. PMID: 24082724.
Article23. Aggarwal P, Saxena S. Stromal differences in odontogenic cysts of a common histopathogenesis but with different biological behavior: a study with picrosirius red and polarizing microscopy. Indian J Cancer. 2011; 48:211–215. PMID: 21768668.
Article24. Sreela KK, Cherian LM, Beena VT, Heera R. Comparison of picrosirius red staining of collagen fibers of Keratocystic odontogenic tumor with odontogenic cysts. IOSR J Dent Med Sci. 2017; 16:45–54.25. Szendröi M, Vajta G, Kovács L, Schaff Z, Lapis K. Polarization colours of collagen fibres: a sign of collagen production activity in fibrotic processes. Acta Morphol Hung. 1984; 32:47–55. PMID: 6431760.26. Bergamini ML, Mardegan AP, DE Rosa CS, Palmieri M, Sarmento DJ, Hiraki KR, Costa AL, HassÉus B, Jonasson P, Braz-Silva PH. Presence of langerhans cells, regulatory T cells (Treg) and mast cells in asymptomatic apical periodontitis. Braz Oral Res. 2020; 34:e108. PMID: 32876121.27. de Oliveira Rodini C, Batista AC, Lara VS. Comparative immunohistochemical study of the presence of mast cells in apical granulomas and periapical cysts: possible role of mast cells in the course of human periapical lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004; 97:59–63. PMID: 14716257.
Article28. Drazić R, Sopta J, Minić AJ. Mast cells in periapical lesions: potential role in their pathogenesis. J Oral Pathol Med. 2010; 39:257–262. PMID: 20359310.
Article29. Fonseca-Silva T, Santos CC, Alves LR, Dias LC, Brito M Jr, De Paula AM, Guimarães AL. Detection and quantification of mast cell, vascular endothelial growth factor, and microvessel density in human inflammatory periapical cysts and granulomas. Int Endod J. 2012; 45:859–864. PMID: 22486765.
Article30. Mahita VN, Manjunatha BS, Shah R, Astekar M, Purohit S, Kovvuru S. Quantification and localization of mast cells in periapical lesions. Ann Med Health Sci Res. 2015; 5:115–118. PMID: 25861530.
Article31. Toskos D, Di Bernardo JF. Periapical lesions: does the size of an endodontic lesion play a role in success and failure? Perio implant advisory 2019 [Internet]. Tulsa, OK: Dental Economics;2020. updated 2020 Jan 2. Available from: https://www.dentaleconomics.com/science-tech/endodontics/article/14074456/periapical-lesions-does-the-size-of-an-endodontic-lesion-play-a-role-in-success-and-failure.32. Thakur SK, Thakur R, Sankhyan A, Patyal A. A conservative approach to treat large periapical lesions: a report of two cases. Indian J Dent Sci. 2019; 11:225–228.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Clinical Study of Periapical Lesions
- Comparison of digital radiometric featuresbetween radicular cysts and periapical granulomas
- Evaluation of ultrasonography as a diagnostic tool in the management of periapical cysts and granulomas: A clinical study
- Differential diagnosis of periapical cyst using collagen birefringence pattern of the cyst wall
- The relationship of radiographic lesion size and characteristics to diagnosis of periapical cysts and granulomas