Cancer Res Treat.
2023 Oct;55(4):1181-1189. 10.4143/crt.2022.1656.
Clinical Outcome of Stereotactic Body Radiotherapy in Patients with Early-Stage Lung Cancer with Ground-Glass Opacity Predominant Lesions: A Single Institution Experience
- Affiliations
-
- 1Departments of Radiation Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Departments of Pulmonary and Critical Care Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2547792
- DOI: http://doi.org/10.4143/crt.2022.1656
Abstract
- Purpose
The detection rate of early-stage lung cancer with ground-glass opacity (GGO) has increased, and stereotactic body radiotherapy (SBRT) has been suggested as an alternative to surgery in inoperable patients. However, reports on treatment results are limited. Therefore, we performed a retrospective study to investigate the clinical outcome after SBRT in patients with early-stage lung cancer with GGO-predominant tumor lesions at a single institution.
Materials and Methods
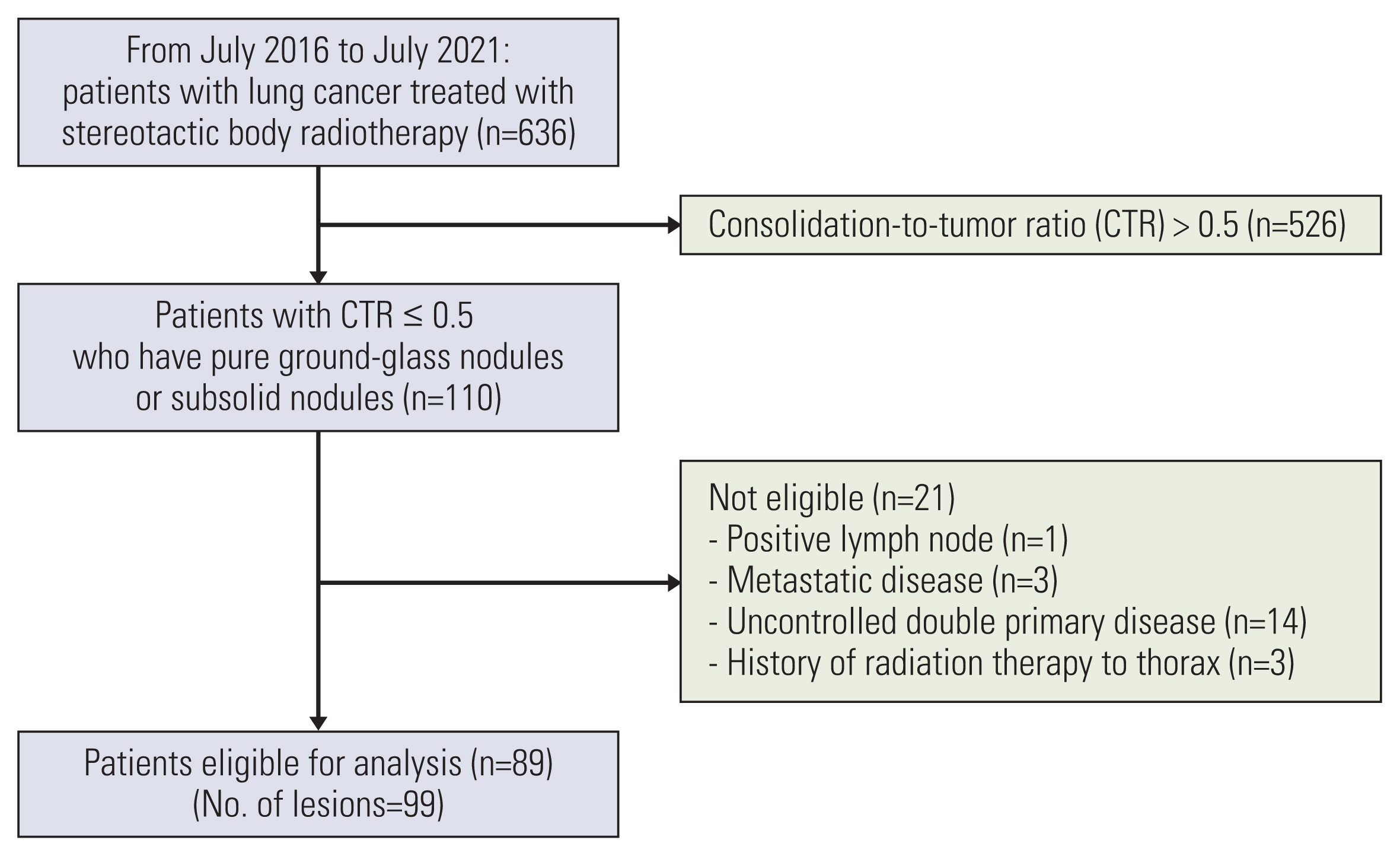
This study included 89 patients with 99 lesions who were treated with SBRT for lung cancer with GGO-predominant lesions that had a consolidation-to-tumor ratio of ≤0.5 at Asan Medical Center between July 2016 and July 2021. A median total dose of 56.0 Gy (range, 48.0–60.0) was delivered using 10.0–15.0 Gy per fraction.
Results
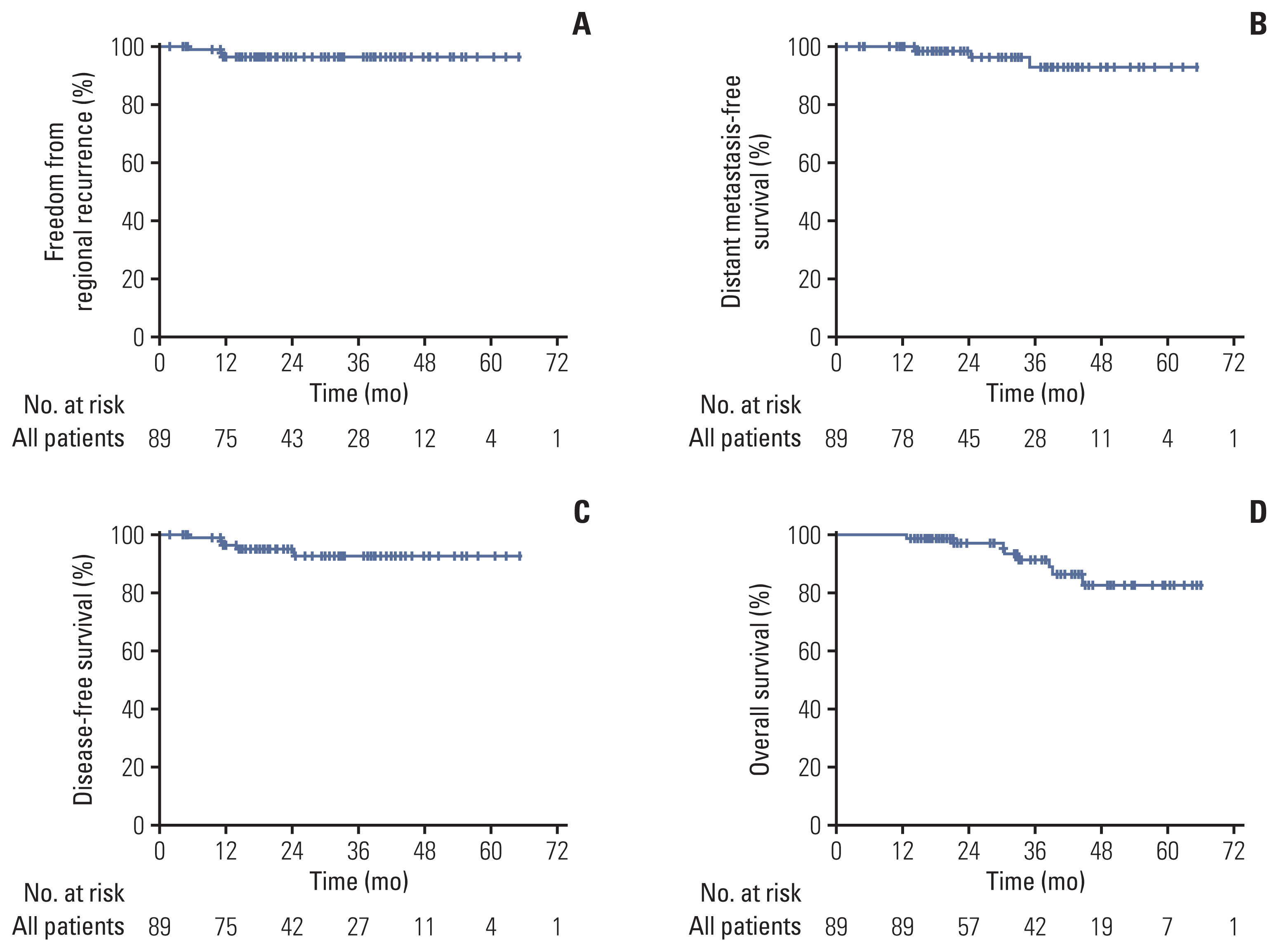
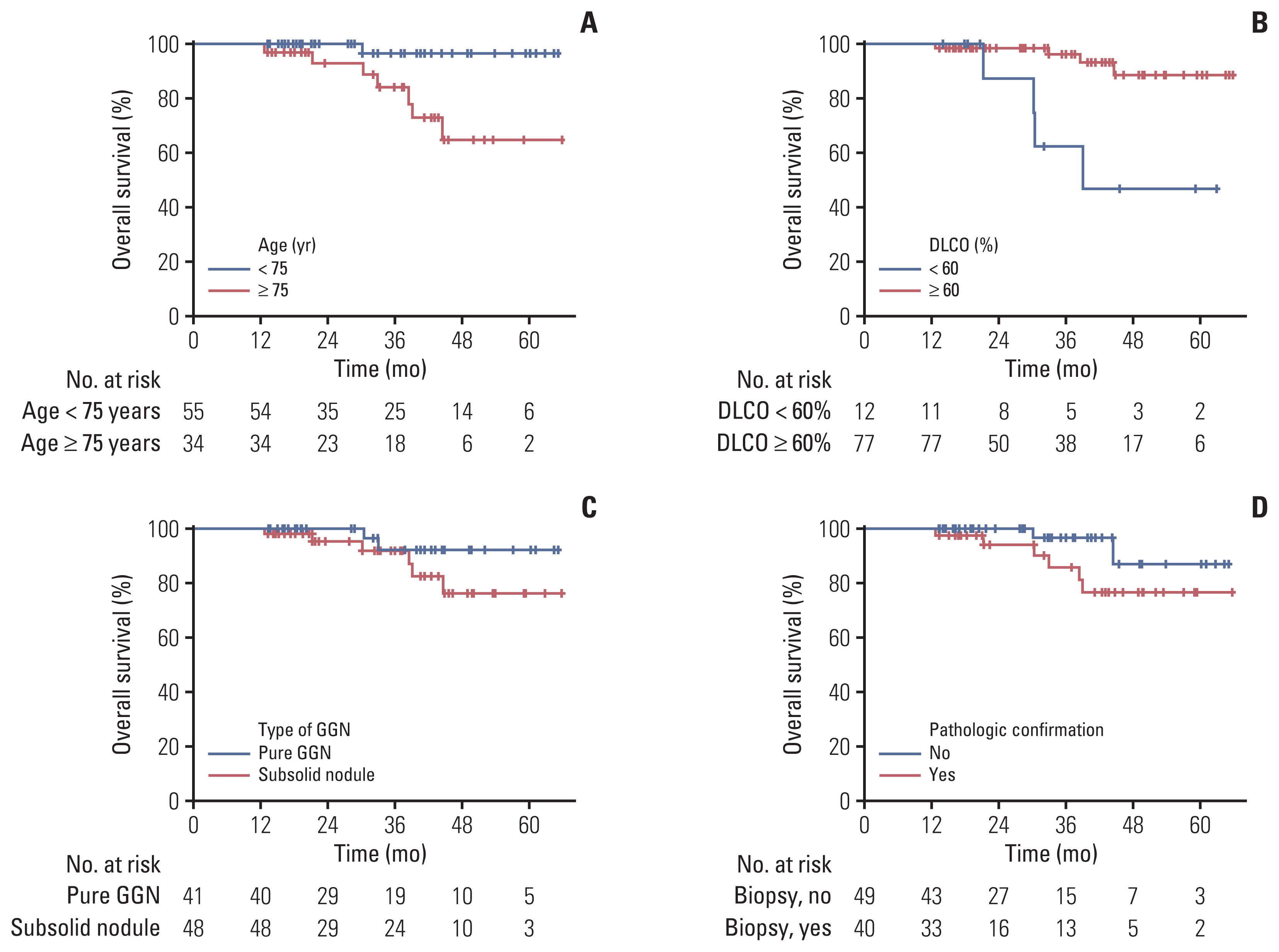
The overall follow-up period for the study was median 33.0 months (range, 9.9 to 65.9 months). There was 100% local control with no recurrences in any of the 99 treated lesions. Three patients had regional recurrences outside of the radiation field, and three had distant metastasis. The 1-year, 3-year, and 5-year overall survival rates were 100.0%, 91.6%, and 82.8%, respectively. Univariate analysis revealed that advanced age and a low level of diffusing capacity of the lungs for carbon monoxide were significantly associated with overall survival. There were no patients with grade ≥3 toxicity.
Conclusion
SBRT is a safe and effective treatment for patients with GGO-predominant lung cancer lesions and is likely to be considered as an alternative to surgery.
Figure
Reference
-
References
1. National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011; 365:395–409.2. Migliore M, Fornito M, Palazzolo M, Criscione A, Gangemi M, Borrata F, et al. Ground glass opacities management in the lung cancer screening era. Ann Transl Med. 2018; 6:90.3. Novello S, Monica V, Serke M, Grohe C, Meyer A, Geissler M, et al. PS01.04 International tailored chemotherapy adjuvant trial: ITACA trial. Final results. J Thorac Oncol. 2021; 16(3 Suppl):S58–9.4. Callister ME, Baldwin DR, Akram AR, Barnard S, Cane P, Draffan J, et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax. 2015; 70:Suppl 2. ii1–54.5. Aokage K, Saji H, Suzuki K, Mizutani T, Katayama H, Shibata T, et al. A non-randomized confirmatory trial of segmentectomy for clinical T1N0 lung cancer with dominant ground glass opacity based on thin-section computed tomography (JCOG1211). Gen Thorac Cardiovasc Surg. 2017; 65:267–72.6. Chang JY, Senan S, Paul MA, Mehran RJ, Louie AV, Balter P, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015; 16:630–7.7. Chang JY, Mehran RJ, Feng L, Verma V, Liao Z, Welsh JW, et al. Stereotactic ablative radiotherapy for operable stage I non-small-cell lung cancer (revised STARS): long-term results of a single-arm, prospective trial with prespecified comparison to surgery. Lancet Oncol. 2021; 22:1448–57.8. Rosenzweig K. Stereotactic body radiation therapy as an alter-native to surgery in early-stage non-small-cell lung cancer. Oncology (Williston Park). 2017; 31:492–8.9. Alcantara P, Martinez BC, Garcia-Esquinas MG, Belaustegui LG, Bustos A. Evaluation of tumor response after stereotactic body radiation therapy for lung cancer: role of 18F-fluorodeoxyglucose positron emission tomography/computed tomography. J Clin Transl Res. 2020; 6:155–67.10. Mikami N, Takeda A, Hashimoto A, Takeda T, Kimura Y, Oku Y, et al. CT findings and treatment outcomes of ground-glass opacity predominant lung cancer after stereotactic body radiotherapy. Clin Lung Cancer. 2022; 23:428–37.11. Nagata I, Ogino T, Arimura T, Yoshiura T. Clinical outcomes of proton beam therapy for ground-glass opacity-type lung cancer. Lung Cancer (Auckl). 2020; 11:105–11.12. Onishi H, Shioyama Y, Matsumoto Y, Shibamoto Y, Miyakawa A, Suzuki G, et al. Stereotactic body radiotherapy in patients with lung tumors composed of mainly ground-glass opacity. J Radiat Res. 2020; 61:426–30.13. Hung JJ, Jeng WJ, Hsu WH, Chou TY, Huang BS, Wu YC. Predictors of death, local recurrence, and distant metastasis in completely resected pathological stage-I non-small-cell lung cancer. J Thorac Oncol. 2012; 7:1115–23.14. Robinson C, Hu C, Machtay M, Newton M, Wu K, Barrett K, et al. P1.18-12 PACIFIC-4/RTOG 3515: phase III study of durvalumab following SBRT for unresected stage I/II, lymph-node negative NSCLC. J Thorac Oncol. 2019; 14(10 Suppl):S630–1.15. Yamashita H, Nakagawa K, Nakamura N, Koyanagi H, Tago M, Igaki H, et al. Exceptionally high incidence of symptomatic grade 2–5 radiation pneumonitis after stereotactic radiation therapy for lung tumors. Radiat Oncol. 2007; 2:21.16. Marks LB, Bentzen SM, Deasy JO, Kong FM, Bradley JD, Vogelius IS, et al. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys. 2010; 76(3 Suppl):S70–6.17. Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ Jr, Wu YL, et al. Lung cancer: current therapies and new targeted treatments. Lancet. 2017; 389:299–311.18. Cho JH, Choi YS, Kim J, Kim HK, Zo JI, Shim YM. Long-term outcomes of wedge resection for pulmonary ground-glass opacity nodules. Ann Thorac Surg. 2015; 99:218–22.19. Tomita N, Okuda K, Osaga S, Miyakawa A, Nakanishi R, Shibamoto Y. Surgery versus stereotactic body radiotherapy for clinical stage I non-small-cell lung cancer: propensity score-matching analysis including the ratio of ground glass nodules. Clin Transl Oncol. 2021; 23:638–47.20. Ye B, Cheng M, Li W, Ge XX, Geng JF, Feng J, et al. Predictive factors for lymph node metastasis in clinical stage IA lung adenocarcinoma. Ann Thorac Surg. 2014; 98:217–23.21. Moon Y, Sung SW, Namkoong M, Park JK. The effectiveness of mediastinal lymph node evaluation in a patient with ground glass opacity tumor. J Thorac Dis. 2016; 8:2617–25.22. Woo CG, Son SM, Lee HC, Han HS, Lee KH, Kim D, et al. Histologic changes in non-small cell lung cancer under various treatments: a comparison of histology and mutation status in serial samples. Cancer Res Treat. 2022; 54:737–43.23. Dahele M, Palma D, Lagerwaard F, Slotman B, Senan S. Radiological changes after stereotactic radiotherapy for stage I lung cancer. J Thorac Oncol. 2011; 6:1221–8.24. Koshy M, Malik R, Weichselbaum RR, Sher DJ. Increasing radiation therapy dose is associated with improved survival in patients undergoing stereotactic body radiation therapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2015; 91:344–50.25. Moreno AC, Fellman B, Hobbs BP, Liao Z, Gomez DR, Chen A, et al. Biologically effective dose in stereotactic body radiotherapy and survival for patients with early-stage NSCLC. J Thorac Oncol. 2020; 15:101–9.26. Hammer MM, Palazzo LL, Kong CY, Hunsaker AR. Cancer risk in subsolid nodules in the National Lung Screening Trial. Radiology. 2019; 293:441–8.27. Lin YH, Hsu HS. Ground glass opacity on chest CT scans from screening to treatment: a literature review. J Chin Med Assoc. 2020; 83:887–90.28. Pierson C, Grinchak T, Sokolovic C, Holland B, Parent T, Bowling M, et al. Response criteria in solid tumors (PERCIST/RECIST) and SUV(max) in early-stage non-small cell lung cancer patients treated with stereotactic body radiotherapy. Radiat Oncol. 2018; 13:34.29. Timmerman RD, Paulus R, Pass HI, Gore EM, Edelman MJ, Galvin J, et al. Stereotactic body radiation therapy for operable early-stage lung cancer: findings from the NRG Oncology RTOG 0618 Trial. JAMA Oncol. 2018; 4:1263–6.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stereotactic Body Radiotherapy for Early Stage Lung Cancer
- Ground-Glass Opacity in Lung Metastasis from Adenocarcinoma of the Stomach: A Case Report
- Stereotactic radiotherapy for early stage non-small cell lung cancer
- The Role of Stereotactic Ablative Radiotherapy for Early-Stage and Oligometastatic Non-small Cell Lung Cancer: Evidence for Changing Paradigms
- The Clinical Approach to Nodular Ground Glass Opacity in the Lung