Anesth Pain Med.
2023 Jul;18(3):233-243. 10.17085/apm.23072.
Predictors of fluid responsiveness in the operating room: a narrative review
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Samsung Medical Center, Seoul, Korea
- KMID: 2547031
- DOI: http://doi.org/10.17085/apm.23072
Abstract
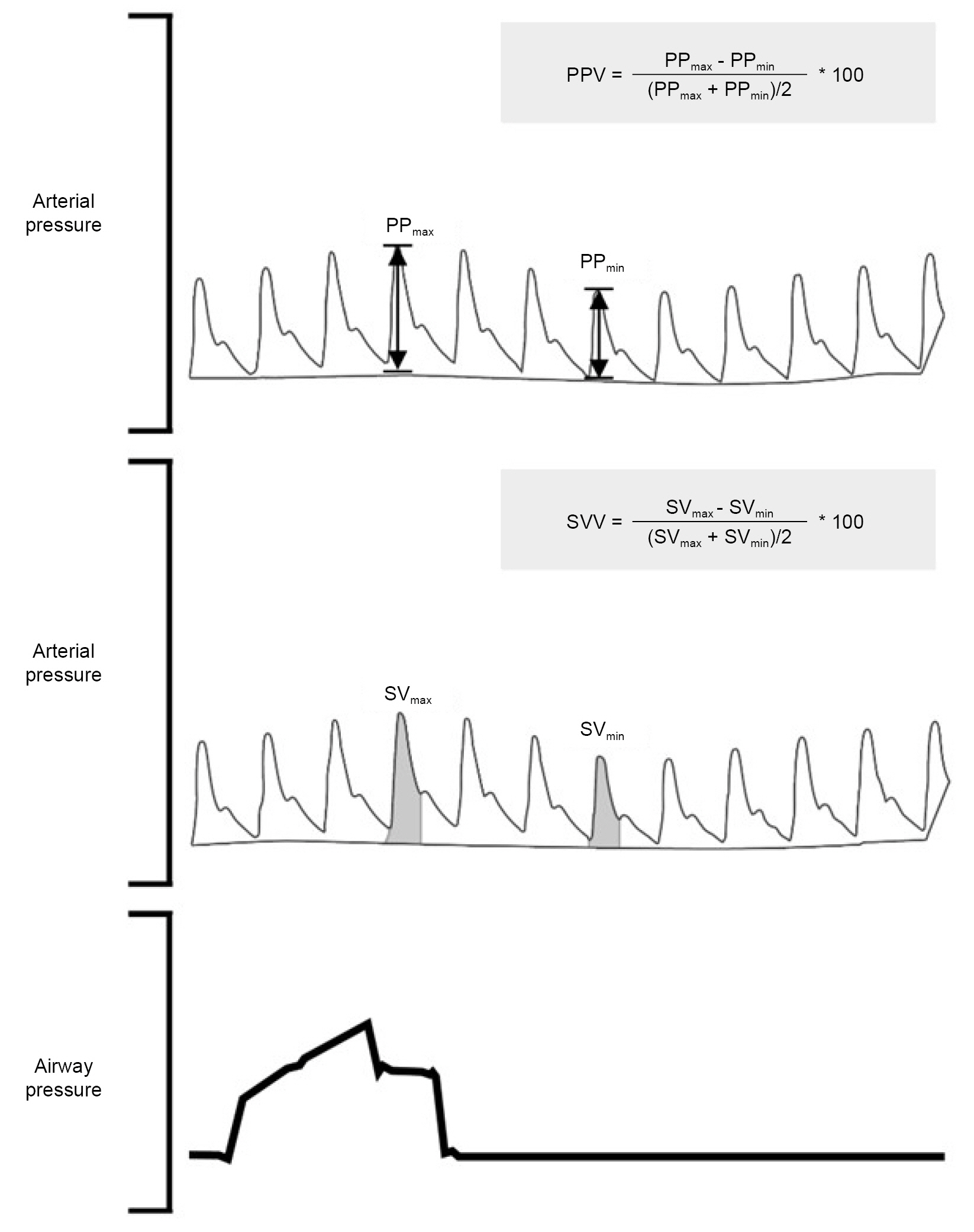
- Prediction of fluid responsiveness has been considered an essential tool for modern fluid management. However, most studies in this field have focused on patients in intensive care unit despite numerous research throughout several decades. Therefore, the present narrative review aims to show the representative method’s feasibility, advantages, and limitations in predicting fluid responsiveness, focusing on the operating room environments. Firstly, we described the predictors of fluid responsiveness based on heart-lung interaction, including pulse pressure and stroke volume variations, the measurement of respiratory variations of inferior vena cava diameter, and the end-expiratory occlusion test and addressed their limitations. Subsequently, the passive leg raising test and mini-fluid challenge tests were also mentioned, which assess fluid responsiveness by mimicking a classic fluid challenge. In the last part of this review, we pointed out the pitfalls of fluid management based on fluid responsiveness prediction, which emphasized the importance of individualized decision-making. Understanding the available representative methods to predict fluid responsiveness and their associated benefits and drawbacks through this review will aid anesthesiologists in choosing the most reliable methods for optimal fluid administration in each patient during anesthesia in the operating room.
Keyword
Figure
Cited by 2 articles
-
Enhancing global recognition: our journey towards Emerging Sources Citation Index indexing
Min Kyoung Kim, Hyun Kang
Anesth Pain Med. 2024;19(4):267-268. doi: 10.17085/apm.24128.A message from the Editor-in-Chief and Editorial Board, 2024: journal metrics and statistics, and appreciation to reviewers
Jun Hyun Kim, Hyun Kang
Anesth Pain Med. 2025;20(1):1-4. doi: 10.17085/apm.25202.
Reference
-
1. Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest. 2002; 121:2000–8.2. Malbrain MLNG, Regenmortel NV, Saugel B, Tavernier BD, Van Gaal PJ, Joannes-Boyau O, et al. Principles of fluid management and stewardship in septic shock: it is time to consider the four D’s and the four phases of fluid therapy. Ann Intensive Care. 2018; 8:66.
Article3. Malbrain MLNG, Langer T, Annane D, Gattinoni L, Elbers P, Hahn RG, et al. Intravenous fluid therapy in the perioperative and critical care setting: executive summary of the International Fluid Academy (IFA). Ann Intensive Care. 2020; 10:64.
Article4. Granado RCD, Mehta RL. Fluid overload in the ICU: evaluation and management. BMC Nephrol. 2016; 17:109.
Article5. Wang N, Jiang L, Zhu B, Wen Y, Xi XM; Beijing Acute Kidney Injury Trial (BAKIT) Workgroup. Fluid balance and mortality in critically ill patients with acute kidney injury: a multicenter prospective epidemiological study. Crit Care. 2015; 19:371.
Article6. Messmer AS, Zingg C, Müller M, Gerber JL, Schefold JC, Pfortmueller CA. Fluid overload and mortality in adult critical care patients—a systematic review and meta-analysis of observational studies. Crit Care Med. 2020; 48:1862–70.
Article7. Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? a systematic review of the literature and the tale of seven mares. Chest. 2008; 134:172–8.
Article8. Marik PE, Lemson J. Fluid responsiveness: an evolution of our understanding. Br J Anaesth. 2014; 112:617–20.
Article9. Tavernier B, Makhotine O, Lebuffe G, Dupont J, Scherpereel P. Systolic pressure variation as a guide to fluid therapy in patients with sepsis-induced hypotension. Anesthesiology. 1998; 89:1313–21.
Article10. Michard F, Boussat S, Chemla D, Anguel N, Mercat A, Lecarpentier Y, et al. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med. 2000; 162:134–8.
Article11. Michard F. Changes in arterial pressure during mechanical ventilation. Anesthesiology. 2005; 103:419–28; quiz 449-5.
Article12. Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med. 2009; 37:2642–7.
Article13. Yang X, Du B. Does pulse pressure variation predict fluid responsiveness in critically ill patients? A systematic review and meta-analysis. Crit Care. 2014; 18:650.
Article14. Zhang Z, Lu B, Sheng X, Jin N. Accuracy of stroke volume variation in predicting fluid responsiveness: a systematic review and meta-analysis. J Anesth. 2011; 25:904–16.
Article15. Teboul JL, Monnet X. Pulse pressure variation and ARDS. Minerva Anestesiol. 2013; 79:398–407.16. De Backer D, Heenen S, Piagnerelli M, Koch M, Vincent JL. Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensive Care Med. 2005; 31:517–23.
Article17. De Waal EE, Rex S, Kruitwagen CL, Kalkman CJ, Buhre WF. Dynamic preload indicators fail to predict fluid responsiveness in open-chest conditions. Crit Care Med. 2009; 37:510–5.
Article18. Jacques D, Bendjelid K, Duperret S, Colling J, Piriou V, Viale JP. Pulse pressure variation and stroke volume variation during increased intra-abdominal pressure: an experimental study. Crit Care. 2011; 15:R33.
Article19. Renner J, Gruenewald M, Quaden R, Hanss R, Meybohm P, Steinfath M, et al. Influence of increased intra-abdominal pressure on fluid responsiveness predicted by pulse pressure variation and stroke volume variation in a porcine model. Crit Care Med. 2009; 37:650–8.
Article20. Myatra SN, Prabu NR, Divatia JV, Monnet X, Kulkarni AP, Teboul JL. The changes in pulse pressure variation or stroke volume variation after a “tidal volume challenge” reliably predict fluid responsiveness during low tidal volume ventilation. Crit Care Med. 2017; 45:415–21.
Article21. Min JJ, Gil NS, Lee JH, Ryu DK, Kim CS, Lee SM. Predictor of fluid responsiveness in the ‘grey zone’: augmented pulse pressure variation through a temporary increase in tidal volume. Br J Anaesth. 2017; 119:50–6.
Article22. Min JJ, Kim TK, Lee JH, Park J, Cho HS, Kim WS, et al. Evaluation of augmented pulse pressure variation using the Valsalva manoeuvre as a predictor of fluid responsiveness under open-chest conditions: A prospective observational study. Eur J Anaesthesiol. 2017; 34:254–61.23. De Backer D, Fagnoul D. Intensive care ultrasound: VI. Fluid responsiveness and shock assessment. Ann Am Thorac Soc. 2014; 11:129–36.
Article24. Feissel M, Michard F, Faller JP, Teboul JL. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med. 2004; 30:1834–7.
Article25. Barbier C, Loubières Y, Schmit C, Hayon J, Ricôme JL, Jardin F, et al. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med. 2004; 30:1740–6.
Article26. Machare-Delgado E, Decaro M, Marik PE. Inferior vena cava variation compared to pulse contour analysis as predictors of fluid responsiveness: a prospective cohort study. J Intensive Care Med. 2011; 26:116–24.
Article27. Bortolotti P, Colling D, Colas V, Voisin B, Dewavrin F, Poissy J, et al. Respiratory changes of the inferior vena cava diameter predict fluid responsiveness in spontaneously breathing patients with cardiac arrhythmias. Ann Intensive Care. 2018; 8:79.
Article28. Bentzer P, Griesdale DE, Boyd J, MacLean K, Sirounis D, Ayas NT. Will this hemodynamically unstable patient respond to a bolus of intravenous fluids? JAMA. 2016; 316:1298–309.
Article29. Vignon P, Repessé X, Bégot E, Léger J, Jacob C, Bouferrache K, et al. Comparison of echocardiographic indices used to predict fluid responsiveness in ventilated patients. Am J Respir Crit Care Med. 2017; 195:1022–32.
Article30. Das SK, Choupoo NS, Pradhan D, Saikia P, Monnet X. Diagnostic accuracy of inferior vena caval respiratory variation in detecting fluid unresponsiveness: a systematic review and meta-analysis. Eur J Anaesthesiol. 2018; 35:831–9.31. Huang H, Shen Q, Liu Y, Xu H, Fang Y. Value of variation index of inferior vena cava diameter in predicting fluid responsiveness in patients with circulatory shock receiving mechanical ventilation: a systematic review and meta-analysis. Crit Care. 2018; 22:204.
Article32. Caplan M, Durand A, Bortolotti P, Colling D, Goutay J, Duburcq T, et al. Measurement site of inferior vena cava diameter affects the accuracy with which fluid responsiveness can be predicted in spontaneously breathing patients: a post hoc analysis of two prospective cohorts. Ann Intensive Care. 2020; 10:168.
Article33. Chiumello D, Chidini G, Calderini E, Colombo A, Crimella F, Brioni M. Respiratory mechanics and lung stress/strain in children with acute respiratory distress syndrome. Ann Intensive Care. 2016; 6:11.
Article34. Gavelli F, Teboul JL, Monnet X. The end-expiratory occlusion test: please, let me hold your breath! Crit Care. 2019; 23:274.
Article35. Monnet X, Osman D, Ridel C, Lamia B, Richard C, Teboul JL. Predicting volume responsiveness by using the end-expiratory occlusion in mechanically ventilated intensive care unit patients. Crit Care Med. 2009; 37:951–6.
Article36. Monnet X, Bleibtreu A, Ferre A, Dres M, Gharbi R, Richard C, et al. Passive leg-raising and end-expiratory occlusion tests perform better than pulse pressure variation in patients with low respiratory system compliance. Crit Care Med. 2012; 40:152–7.
Article37. Biais M, Larghi M, Henriot J, De Courson H, Sesay M, Nouette-Gaulain K. End-expiratory occlusion test predicts fluid responsiveness in patients with protective ventilation in the operating room. Anesth Analg. 2017; 125:1889–95.
Article38. Guinot PG, Godart J, De Broca B, Bernard E, Lorne E, Dupont H. End-expiratory occlusion manoeuvre does not accurately predict fluid responsiveness in the operating theatre. Br J Anaesth. 2014; 112:1050–4.
Article39. Weil G, Motamed C, Monnet X, Eghiaian A, Le Maho AL. End-expiratory occlusion test to predict fluid responsiveness is not suitable for laparotomic surgery. Anesth Analg. 2020; 130:151–8.
Article40. Messina A, Montagnini C, Cammarota G, Giuliani F, Muratore L, Baggiani M, et al. Assessment of fluid responsiveness in prone neurosurgical patients undergoing protective ventilation: role of dynamic indices, tidal volume challenge, and end-expiratory occlusion test. Anesth Analg. 2020; 130:752–61.
Article41. Edgcombe H, Carter K, Yarrow S. Anaesthesia in the prone position. Br J Anaesth. 2008; 100:165–83.
Article42. Wadsworth R, Anderton JM, Vohra A. The effect of four different surgical prone positions on cardiovascular parameters in healthy volunteers. Anaesthesia. 1996; 51:819–22.
Article43. Jabot J, Teboul JL, Richard C, Monnet X. Passive leg raising for predicting fluid responsiveness: importance of the postural change. Intensive Care Med. 2009; 35:85–90.
Article44. Boulain T, Achard JM, Teboul JL, Richard C, Perrotin D, Ginies G. Changes in BP induced by passive leg raising predict response to fluid loading in critically ill patients. Chest. 2002; 121:1245–52.
Article45. Monnet X, Marik P, Teboul JL. Passive leg raising for predicting fluid responsiveness: a systematic review and meta-analysis. Intensive Care Med. 2016; 42:1935–47.
Article46. Mallat J, Fischer MO, Granier M, Vinsonneau C, Jonard M, Mahjoub Y, et al. Passive leg raising-induced changes in pulse pressure variation to assess fluid responsiveness in mechanically ventilated patients: a multicentre prospective observational study. Br J Anaesth. 2022; 129:308–16.
Article47. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Executive summary: surviving sepsis campaign: international guidelines for the management of sepsis and septic shock 2021. Crit Care Med. 2021; 49:1974–82.48. Monnet X, Shi R, Teboul JL. Prediction of fluid responsiveness. What’s new? Ann Intensive Care. 2022; 12:46.
Article49. El Hadouti Y, Valencia L, Becerra A, Rodríguez-Pérez A, Vincent JL. Echocardiography and passive leg raising in the postoperative period: a prospective observational study. Eur J Anaesthesiol. 2017; 34:748–54.50. Beurton A, Teboul JL, Gavelli F, Gonzalez FA, Girotto V, Galarza L, et al. The effects of passive leg raising may be detected by the plethysmographic oxygen saturation signal in critically ill patients. Crit Care. 2019; 23:19.
Article51. Galarza L, Mercado P, Teboul JL, Girotto V, Beurton A, Richard C, et al. Estimating the rapid haemodynamic effects of passive leg raising in critically ill patients using bioreactance. Br J Anaesth. 2018; 121:567–73.
Article52. Kupersztych-Hagege E, Teboul JL, Artigas A, Talbot A, Sabatier C, Richard C, et al. Bioreactance is not reliable for estimating cardiac output and the effects of passive leg raising in critically ill patients. Br J Anaesth. 2013; 111:961–6.
Article53. Monnet X, Teboul JL. Passive leg raising: five rules, not a drop of fluid! Critical Care. 2015; 19:18.
Article54. Chacko CJ, Wise MP, Frost PJ. Passive leg raising and compression stockings: a note of caution. Crit Care. 2015; 19:237.
Article55. Beurton A, Teboul JL, Girotto V, Galarza L, Anguel N, Richard C, et al. Intra-abdominal hypertension is responsible for false negatives to the passive leg raising test. Crit Care Med. 2019; 47:e639–47.
Article56. Weil MH, Henning RJ. New concepts in the diagnosis and fluid treatment of circulatory shock: thirteenth annual becton, dickinson and company oscar schwidetsky memorial lecture. Anesth Analg. 1979; 58:124–32.57. Muller L, Toumi M, Bousquet PJ, Riu-Poulenc B, Louart G, Candela D, et al. An increase in aortic blood flow after an infusion of 100 ml colloid over 1 minute can predict fluid responsiveness: the mini-fluid challenge study. Anesthesiology. 2011; 115:541–7.58. Wu Y, Zhou S, Zhou Z, Liu B. A 10-second fluid challenge guided by transthoracic echocardiography can predict fluid responsiveness. Crit Care. 2014; 18:R108.
Article59. Smorenberg A, Cherpanath TGV, Geerts BF, De Wilde RBP, Jansen JRC, Maas JJ, et al. A mini-fluid challenge of 150 mL predicts fluid responsiveness using ModelflowR pulse contour cardiac output directly after cardiac surgery. J Clin Anesth. 2018; 46:17–22.
Article60. Guinot PG, Bernard E, Defrancq F, Petiot S, Majoub Y, Dupont H, et al. Mini-fluid challenge predicts fluid responsiveness during spontaneous breathing under spinal anaesthesia: an observational study. Eur J Anaesthesiol. 2015; 32:645–9.61. Biais M, De Courson H, Lanchon R, Pereira B, Bardonneau G, Griton M, et al. Mini-fluid challenge of 100 ml of crystalloid predicts fluid responsiveness in the operating room. Anesthesiology. 2017; 127:450–6.62. Messina A, Dell'Anna A, Baggiani M, Torrini F, Maresca GM, Bennett V, et al. Functional hemodynamic tests: a systematic review and a metanalysis on the reliability of the end-expiratory occlusion test and of the mini-fluid challenge in predicting fluid responsiveness. Crit Care. 2019; 23:264.
Article63. Messina A, Lionetti G, Foti L, Bellotti E, Marcomini N, Cammarota G, et al. Mini fluid chAllenge aNd End-expiratory occlusion test to assess flUid responsiVEness in the opeRating room (MANEUVER study): a multicentre cohort study. Eur J Anaesthesiol. 2021; 38:422–31.64. Enevoldsen J, Scheeren TWL, Berg JM, Vistisen ST. Existing fluid responsiveness studies using the mini‐fluid challenge may be misleading: methodological considerations and simulations. Acta Anaesthesiol Scand. 2022; 66:17–24.
Article65. Monnet X, Marik PE, Teboul JL. Prediction of fluid responsiveness: an update. Ann Intensive Care. 2016; 6:111.
Article66. Ray P, Le Manach Y, Riou B, Houle TT. Statistical evaluation of a biomarker. Anesthesiology. 2010; 112:1023–40.
Article67. Cannesson M, Le Manach Y, Hofer CK, Goarin JP, Lehot JJ, Vallet B, et al. Assessing the diagnostic accuracy of pulse pressure variations for the prediction of fluid responsiveness: a “gray zone” approach. Anesthesiology. 2011; 115:231–41.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pulse pressure variation and stroke volume variation to predict fluid responsiveness in patients undergoing carotid endarterectomy
- Role of carotid corrected flow time and peak velocity variation in predicting fluid responsiveness: a systematic review and meta-analysis
- Identification of Nursing Interventions in the Operating Room using the Perioperative Nursing Data Set(PNDS)
- Factors Influencing Patient Safety Management Activities among General Hospital Operating Room Nurses
- The Impact of Safety Climate and Fatigue on Safety Performance of Operating Room Nurses